This document discusses soft tissue sarcomas (STS), including:
- Incidence rates in the US and Egypt. Radiation therapy is a risk factor.
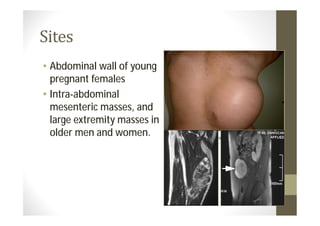
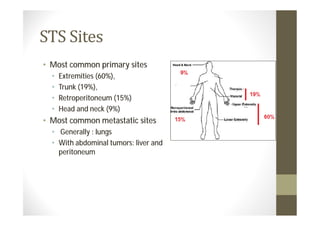
- Common primary and metastatic sites vary by tumor type.
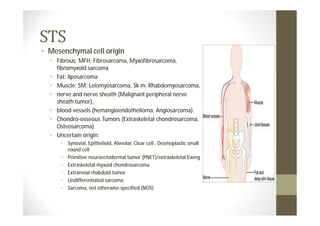
- STS originate from mesenchymal cells and include many subtypes.
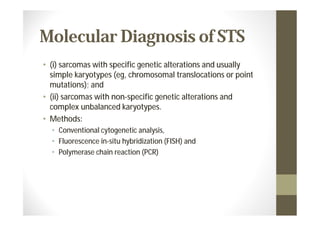
- Diagnosis involves biopsy, imaging, and genetic testing to identify specific mutations in certain sarcoma subtypes.
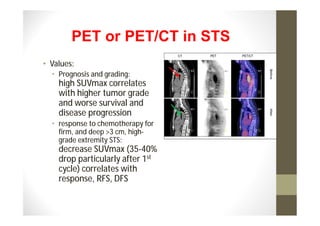
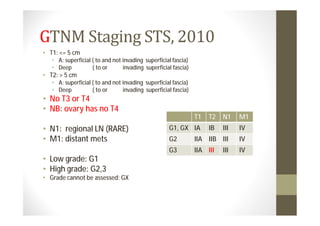
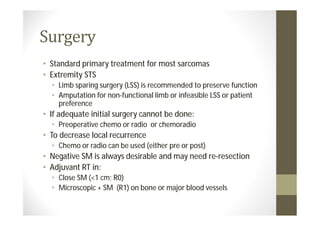
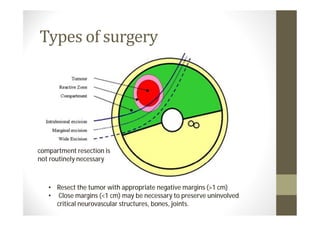
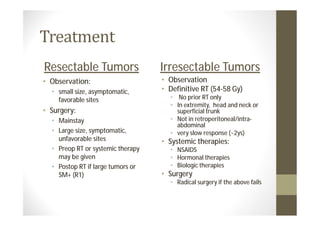
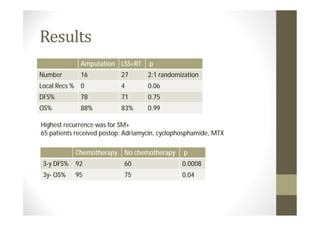
- Treatment depends on grade and stage but commonly involves surgery with or without chemotherapy and/or radiation therapy. Outcomes vary significantly by histology, grade, and other factors.



![• EWSR1-ATF1 in clear cell sarcoma,
• TLS-CHOP (also known as FUS-DDIT3) in myxoid or round cell
liposarcoma,
• SS18-SSX (SS18-SSX1 or SS18-SSX2) in synovial sarcoma, and
• PAX-FOXO1 (PAX3-FOXO1 or PAX7-FOXO1) in alveolar
rhabdomyosarcoma].](https://image.slidesharecdn.com/softtissuesarcomasts2-130324145642-phpapp02/85/Soft-tissue-sarcoma-sts-7-320.jpg)