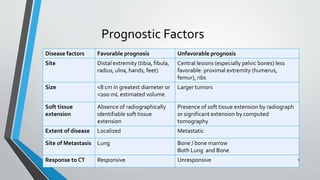
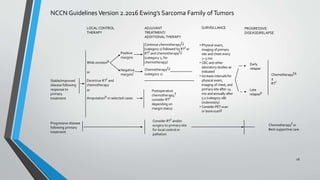
This document provides information on the management of Ewing's Sarcoma, including:
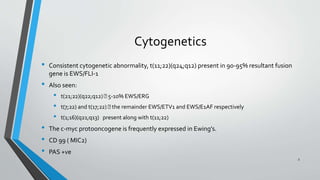
- Ewing's Sarcoma is identified by the translocation t(11;22) in 90-95% of cases.
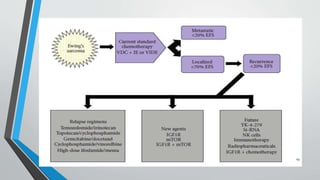
- Metastases most commonly spread to the lungs, bones, and bone marrow. Nearly all patients have micrometastases at diagnosis.
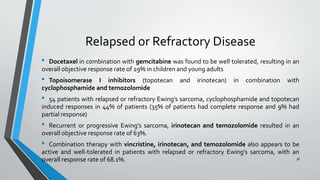
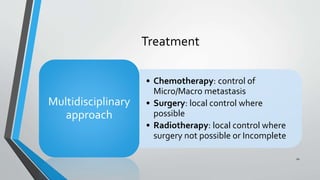
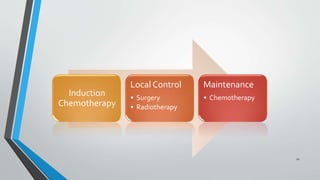
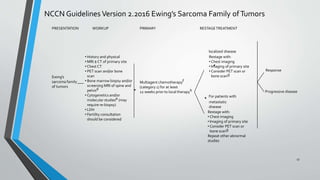
- Treatment involves induction chemotherapy followed by local control with surgery or radiotherapy and maintenance chemotherapy.
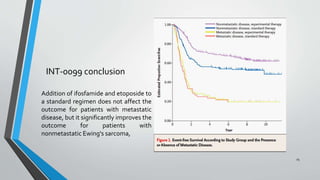
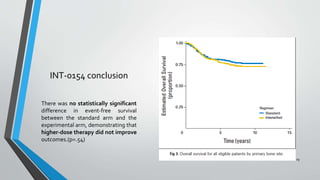
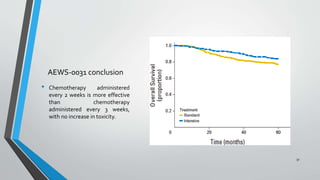
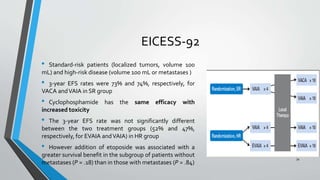
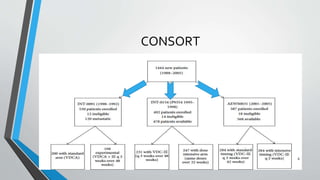
- Several clinical trials have evaluated chemotherapy regimens and dosing schedules, with INT-0099 establishing VDC/IE as the standard of care and AEWS-0031 showing improved outcomes with interval-compressed chemotherapy.












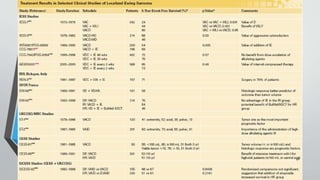
![IESS-II: Multimodal therapy for the management
of nonpelvic, localized Ewing's sarcoma of bone:
intergroup study IESS-II.
• 214 patients
• Adriamycin, cyclophosphamide, vincristine, and dactinomycin by either a high-
dose intermittent method (treatment [trt] 1) or a moderate-dose continuous
method (trt 2) similar to the four-drug arm of IESS-I
• The overall outcome was significantly better on trt 1 than on trt 2
• Treatment failure for both treatment groups was the development of metastatic
disease (lung)
• High-dose intermittent ofVACA and 3 week cycle has better outcome
22](https://image.slidesharecdn.com/ewingssarcomamanagement-160517121743/85/Ewings-sarcoma-management-Chemotherapy-trials-22-320.jpg)