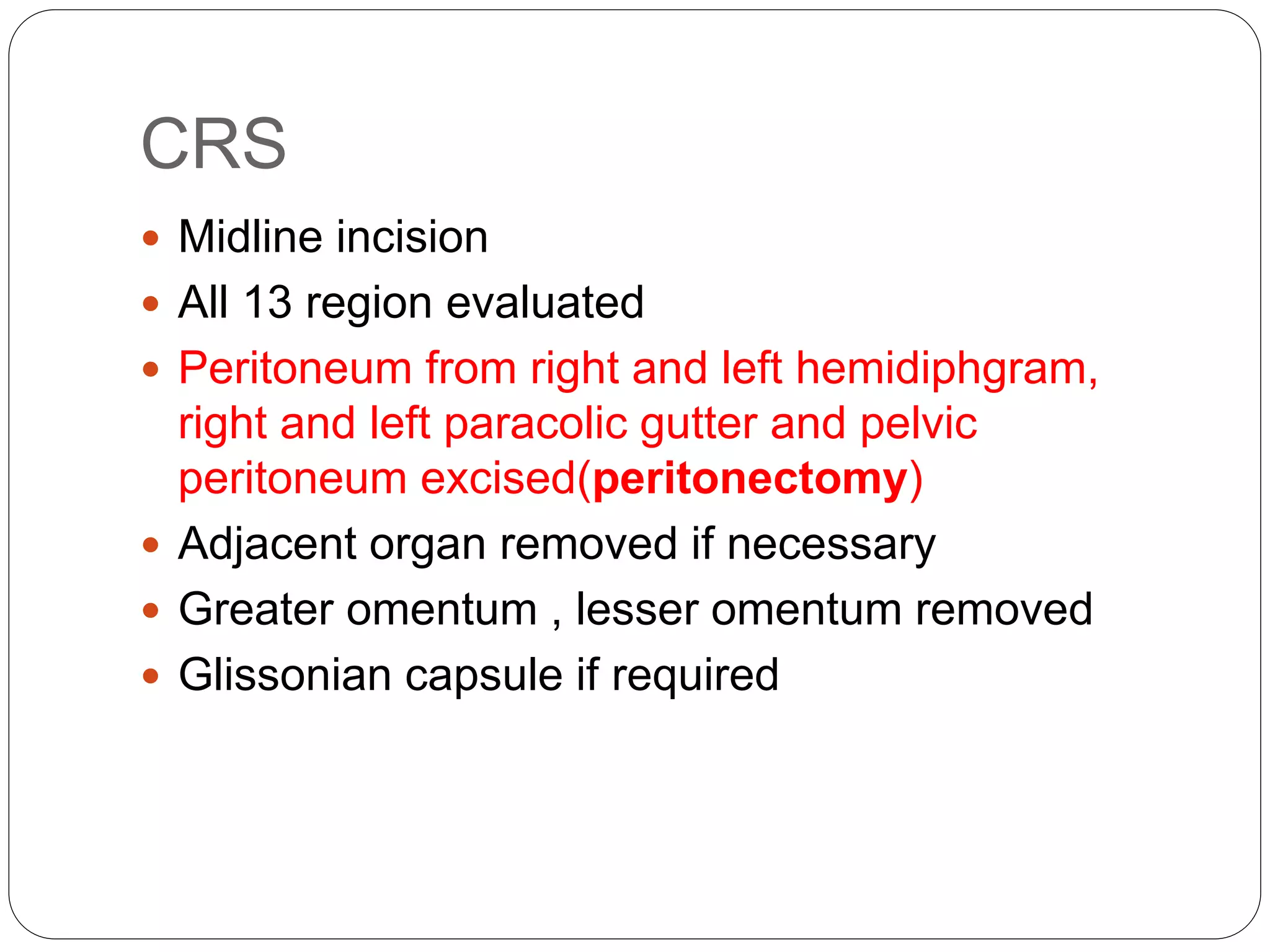
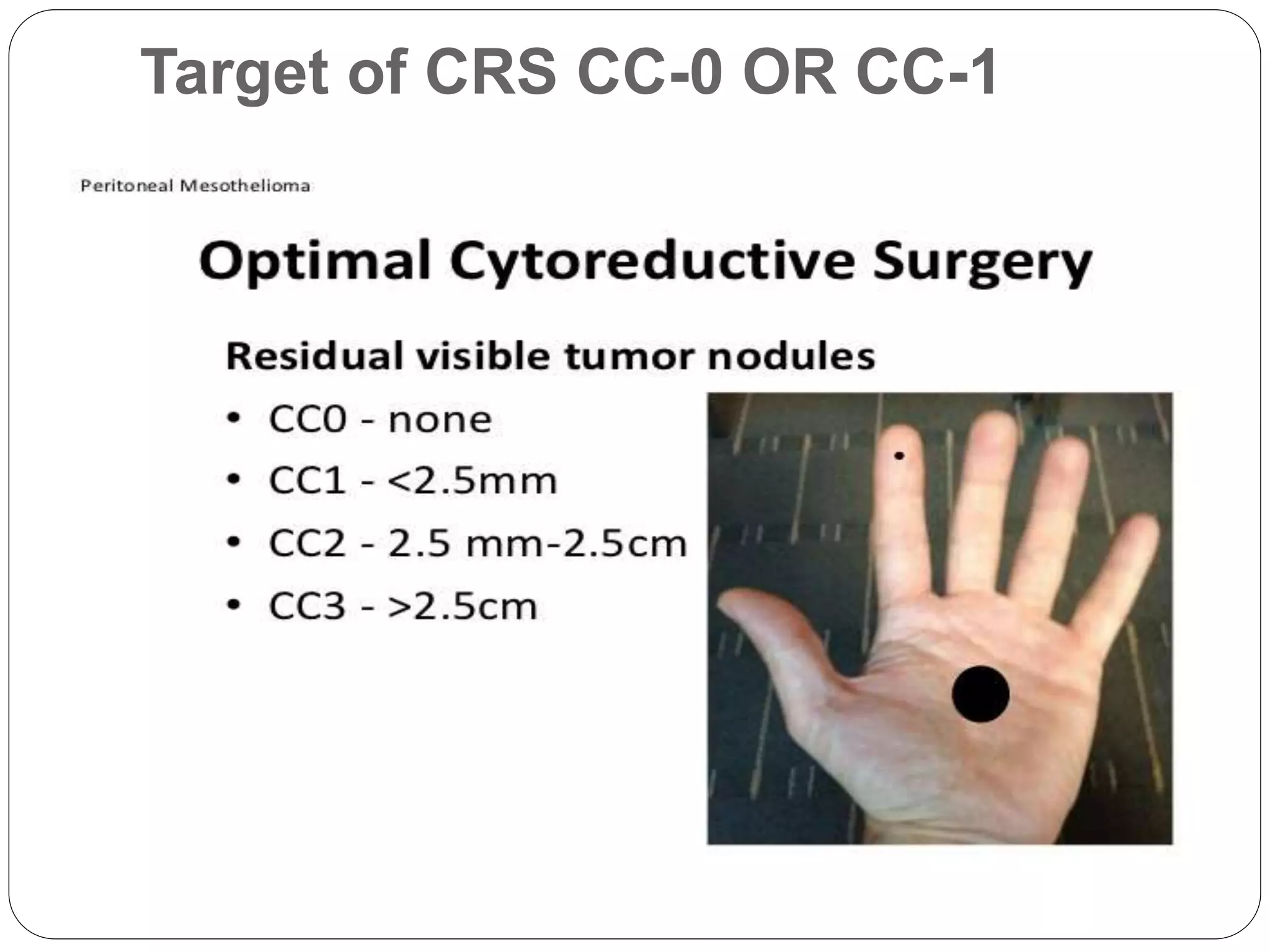
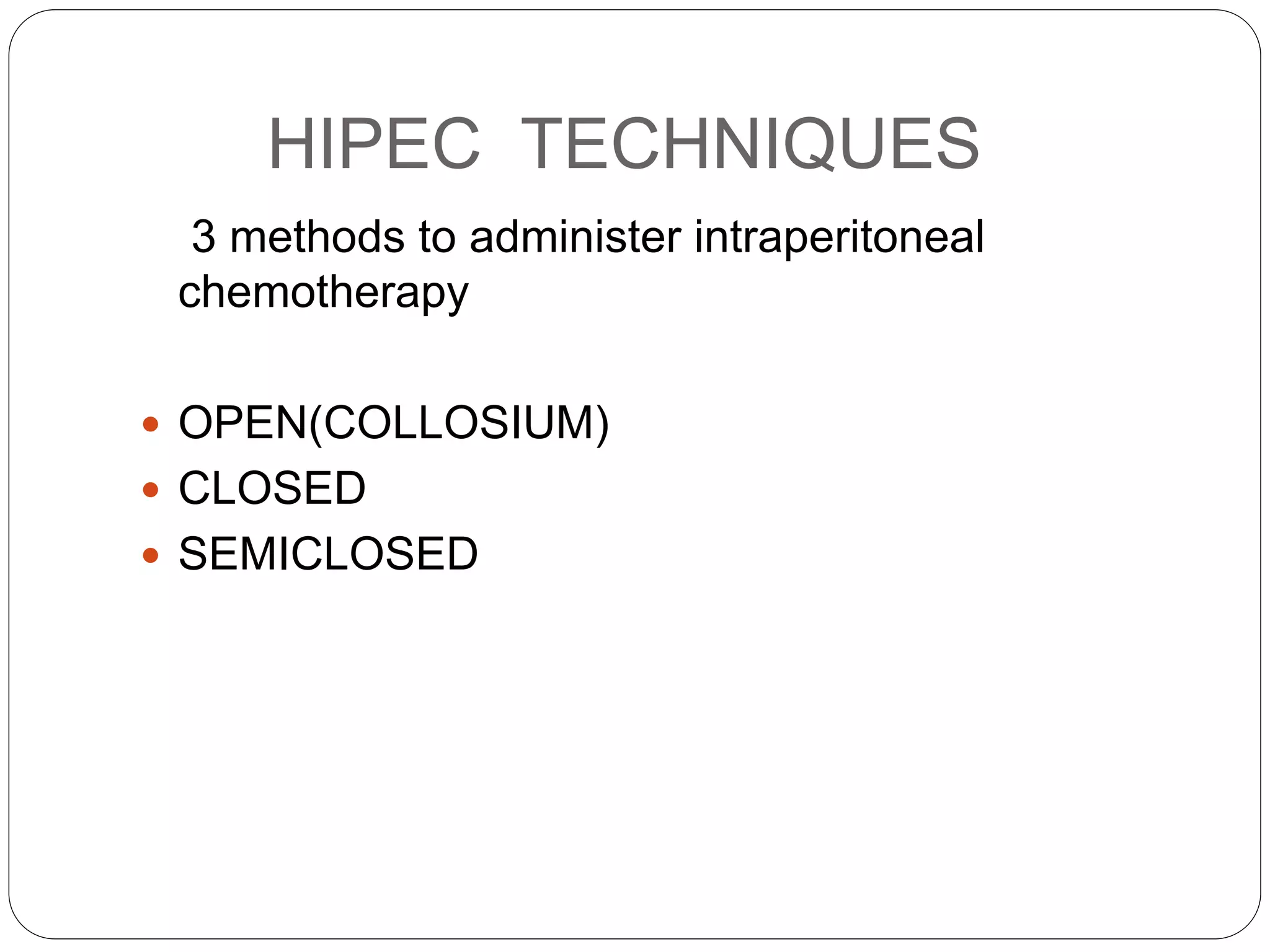
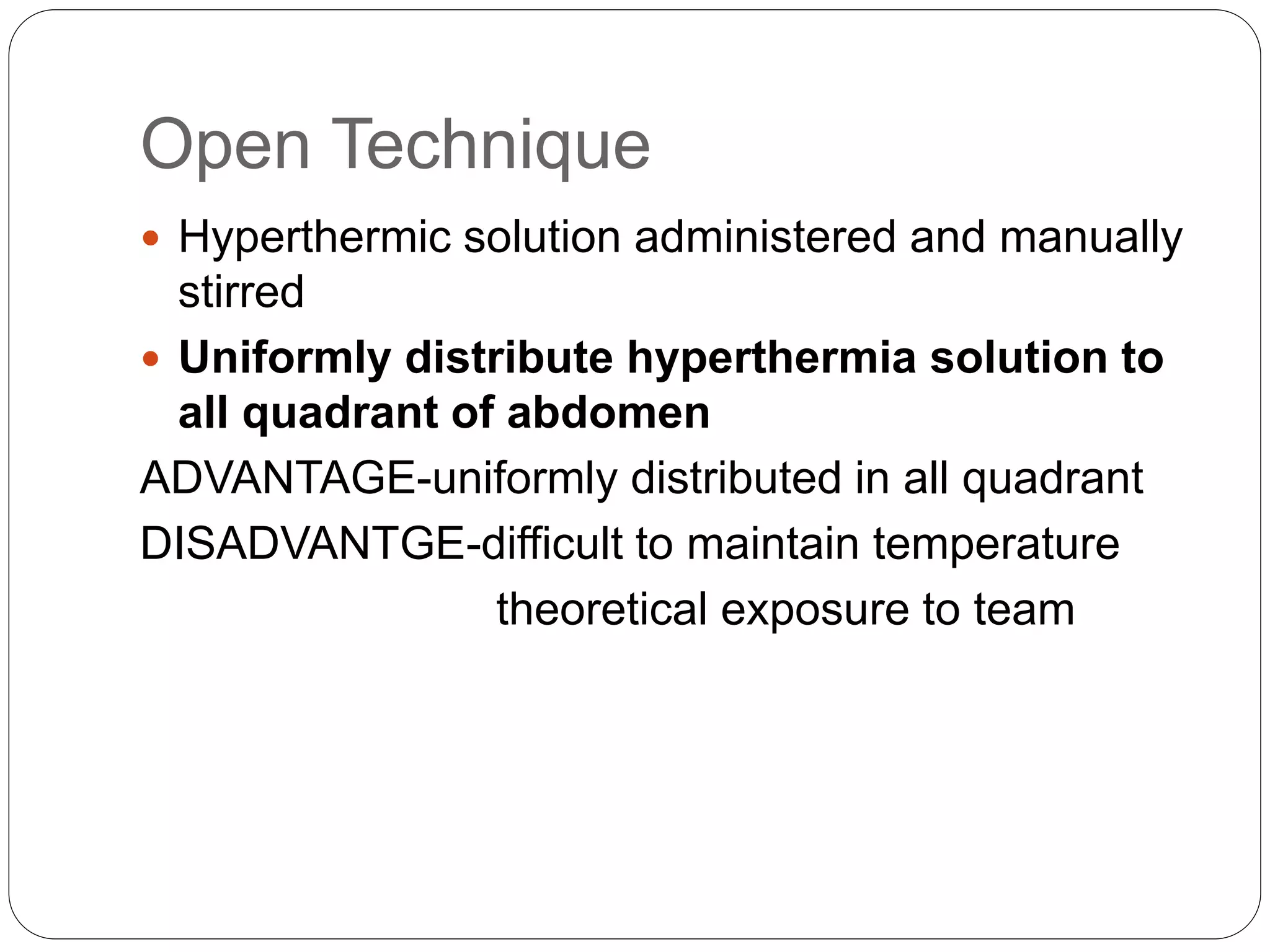
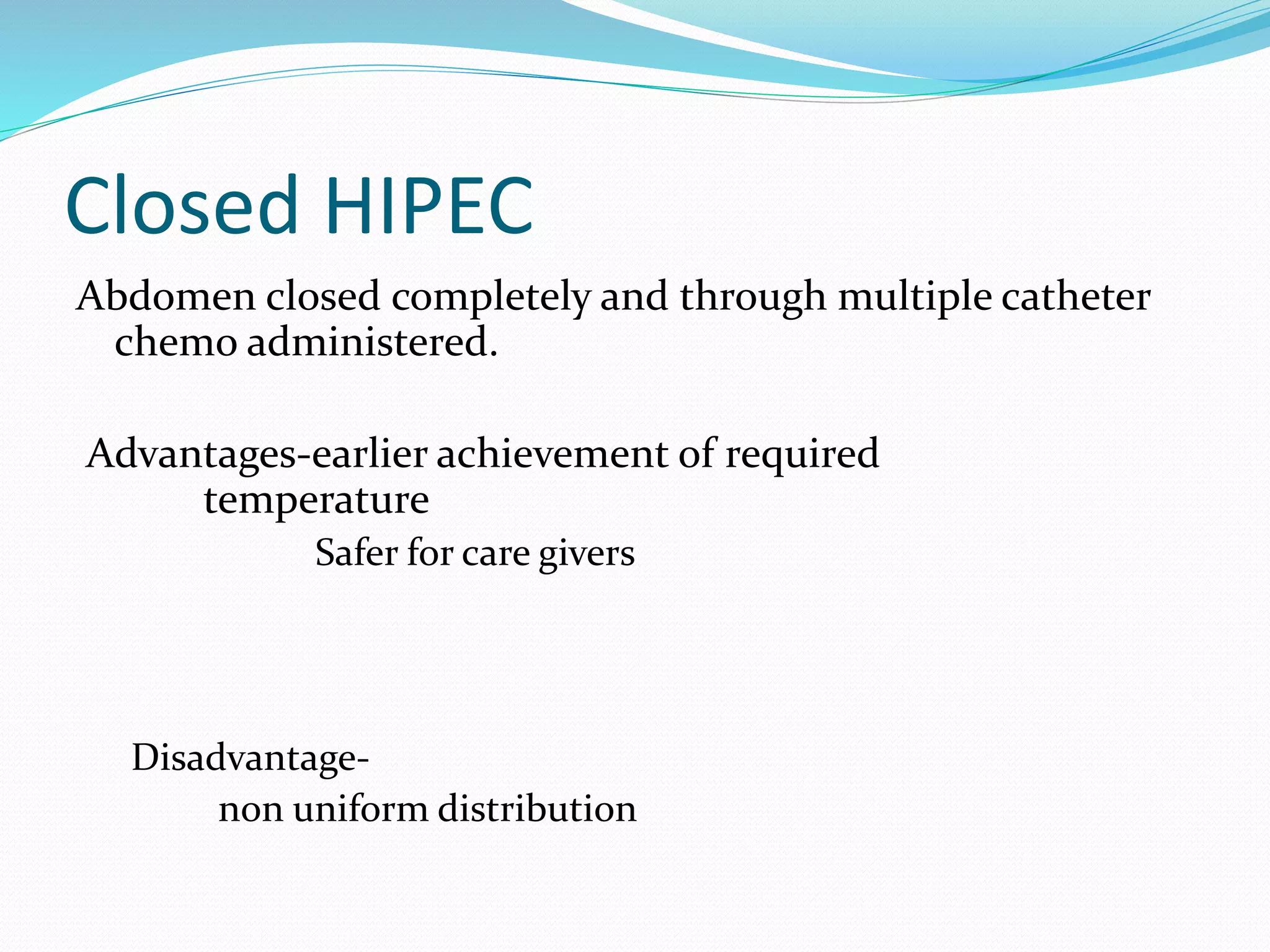
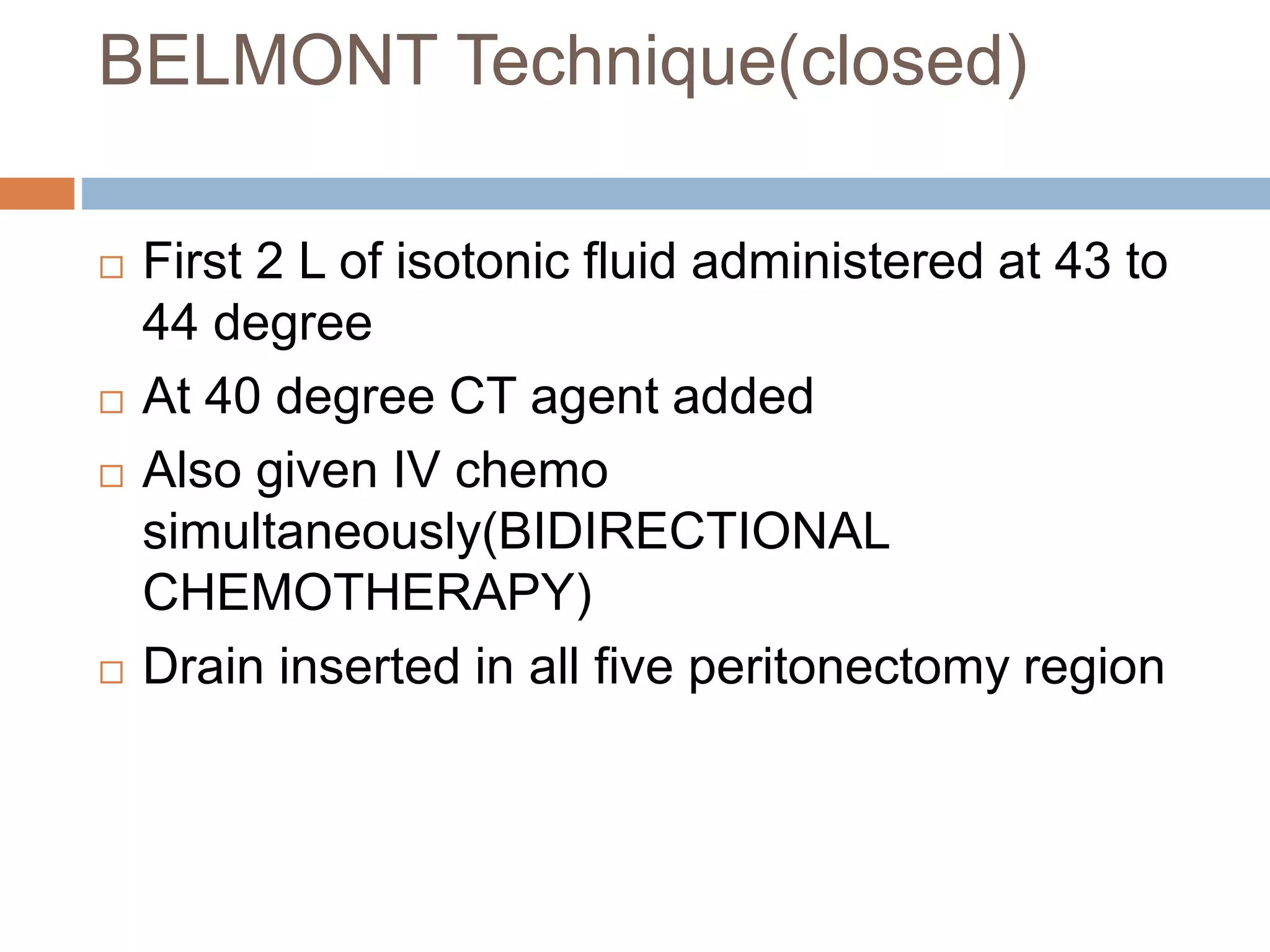
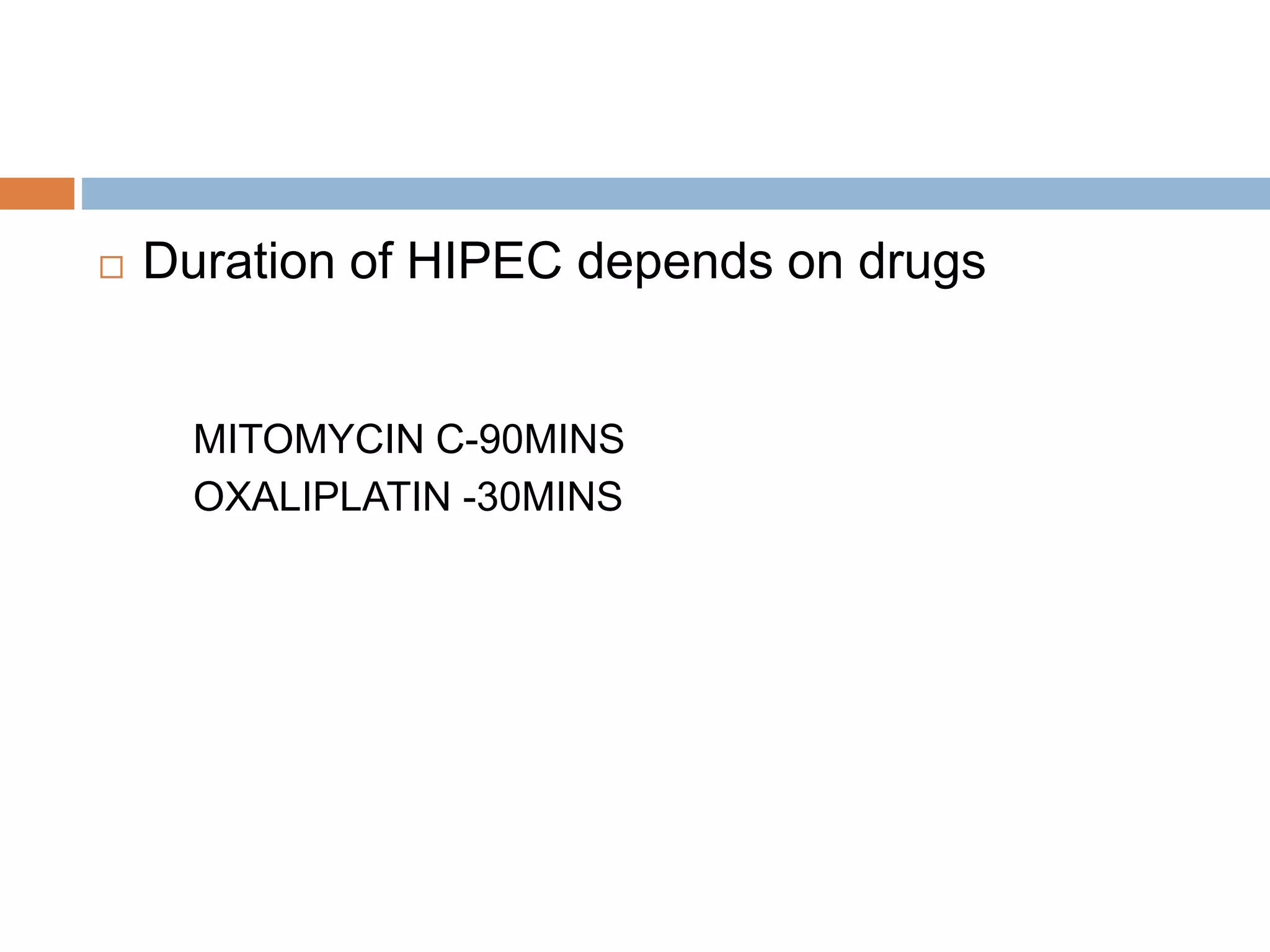
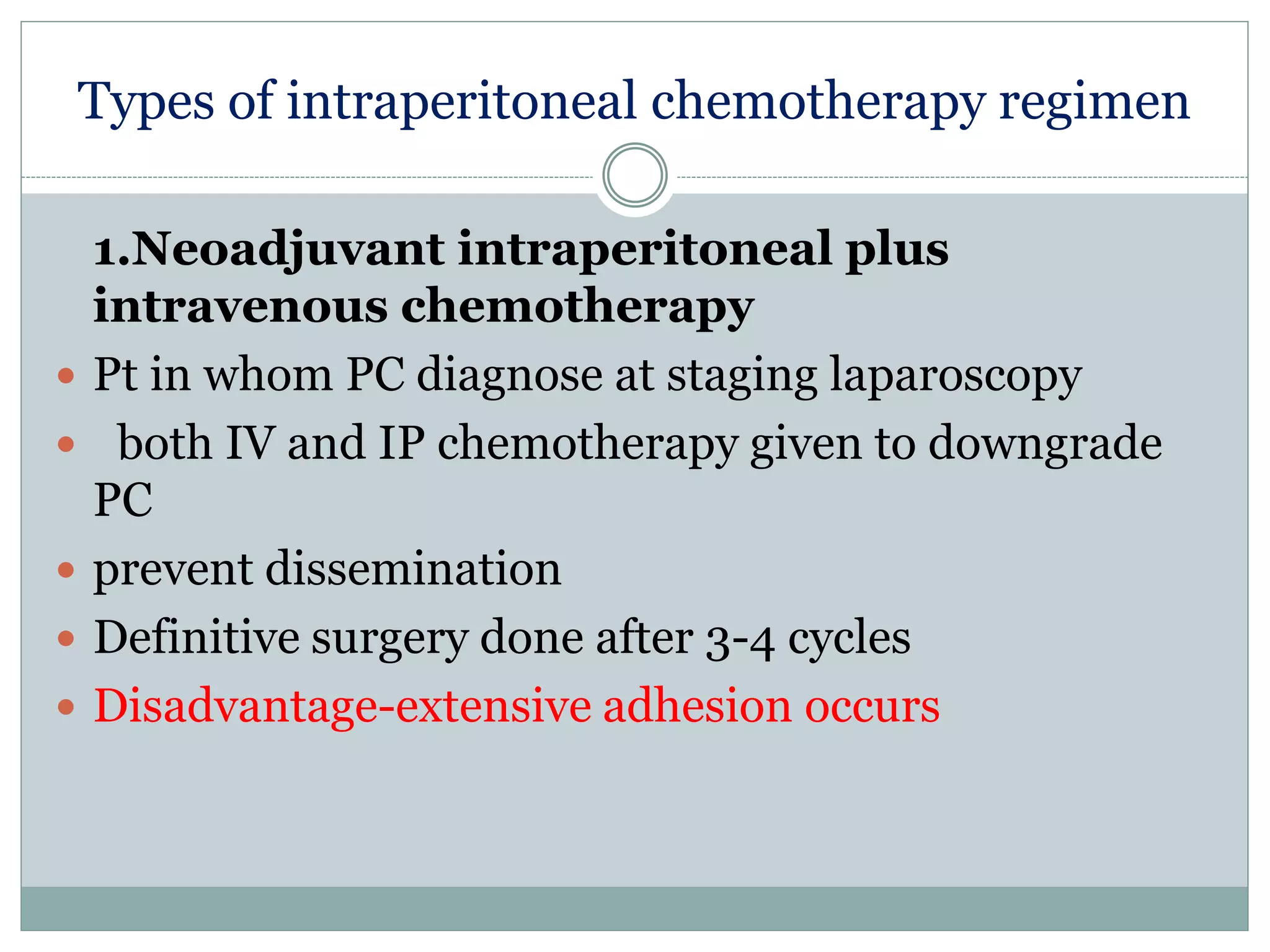
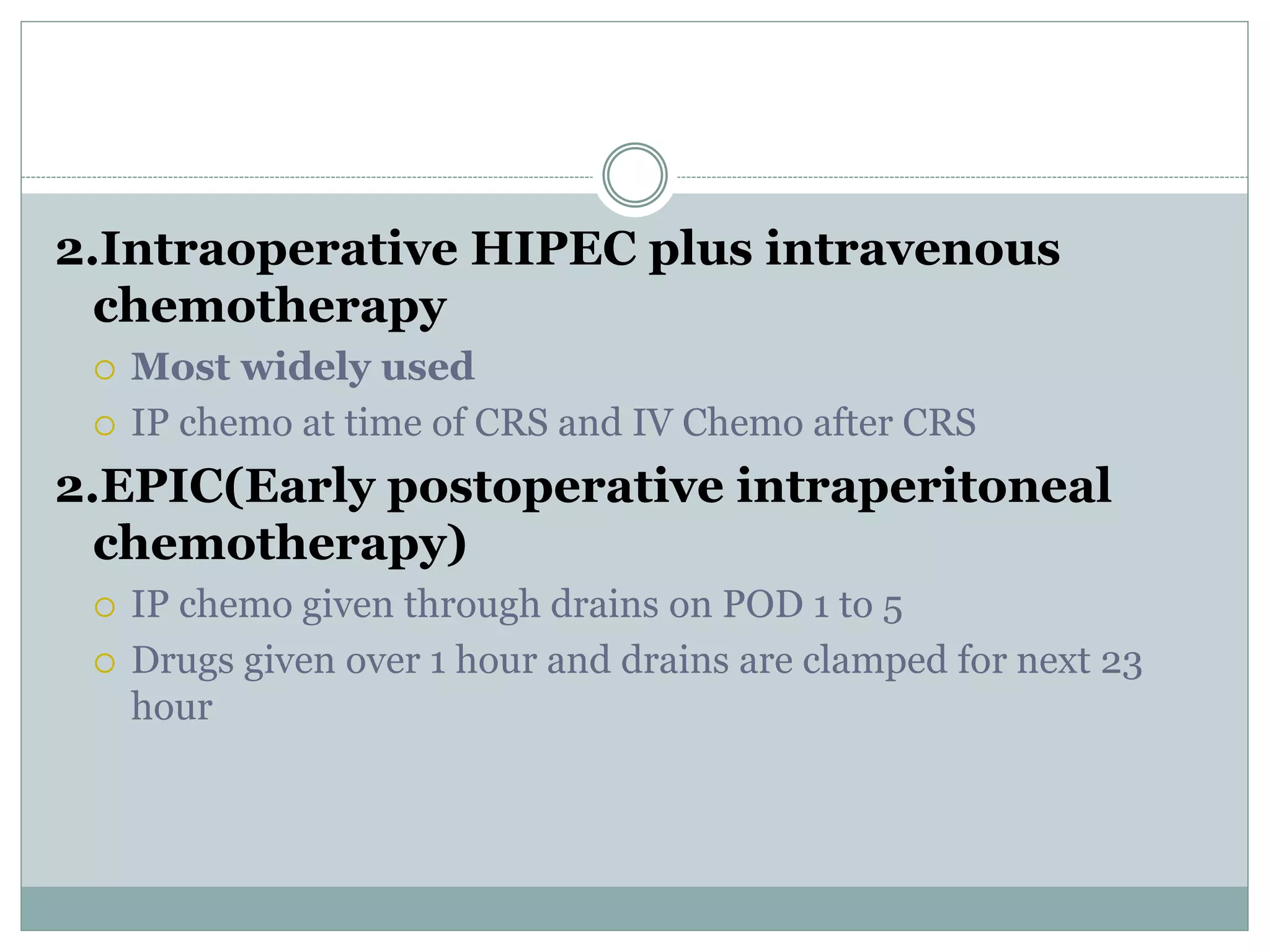
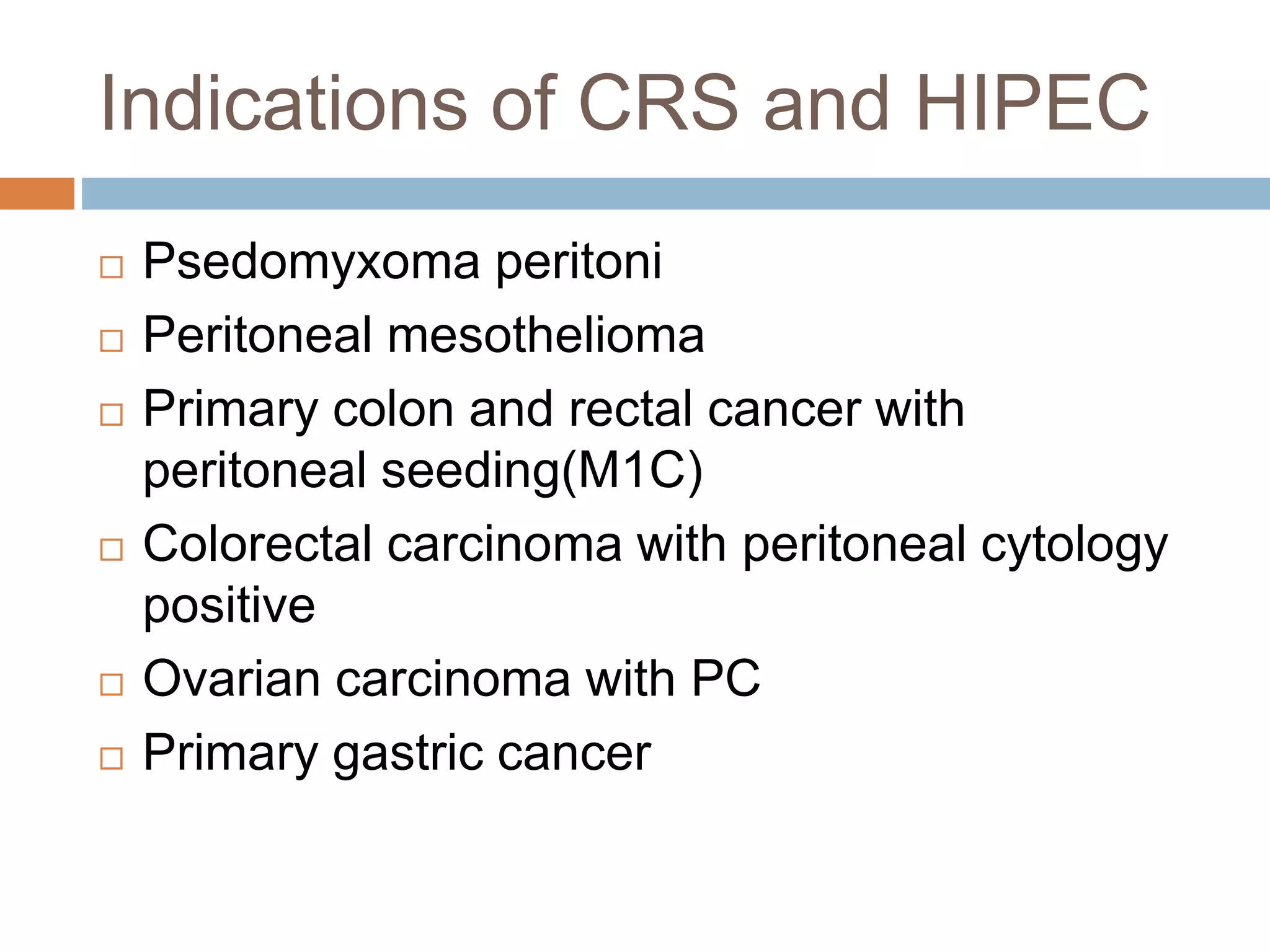
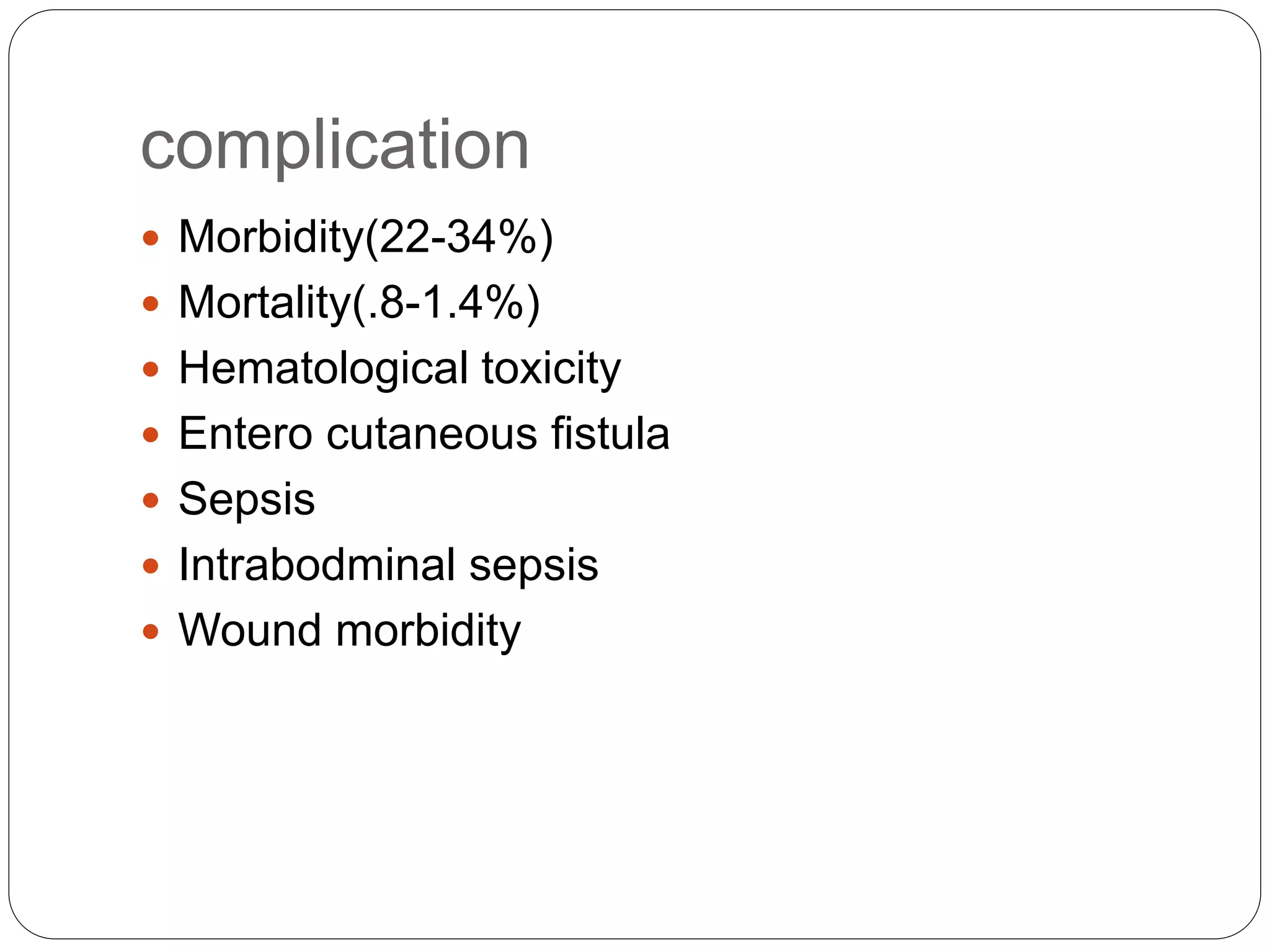
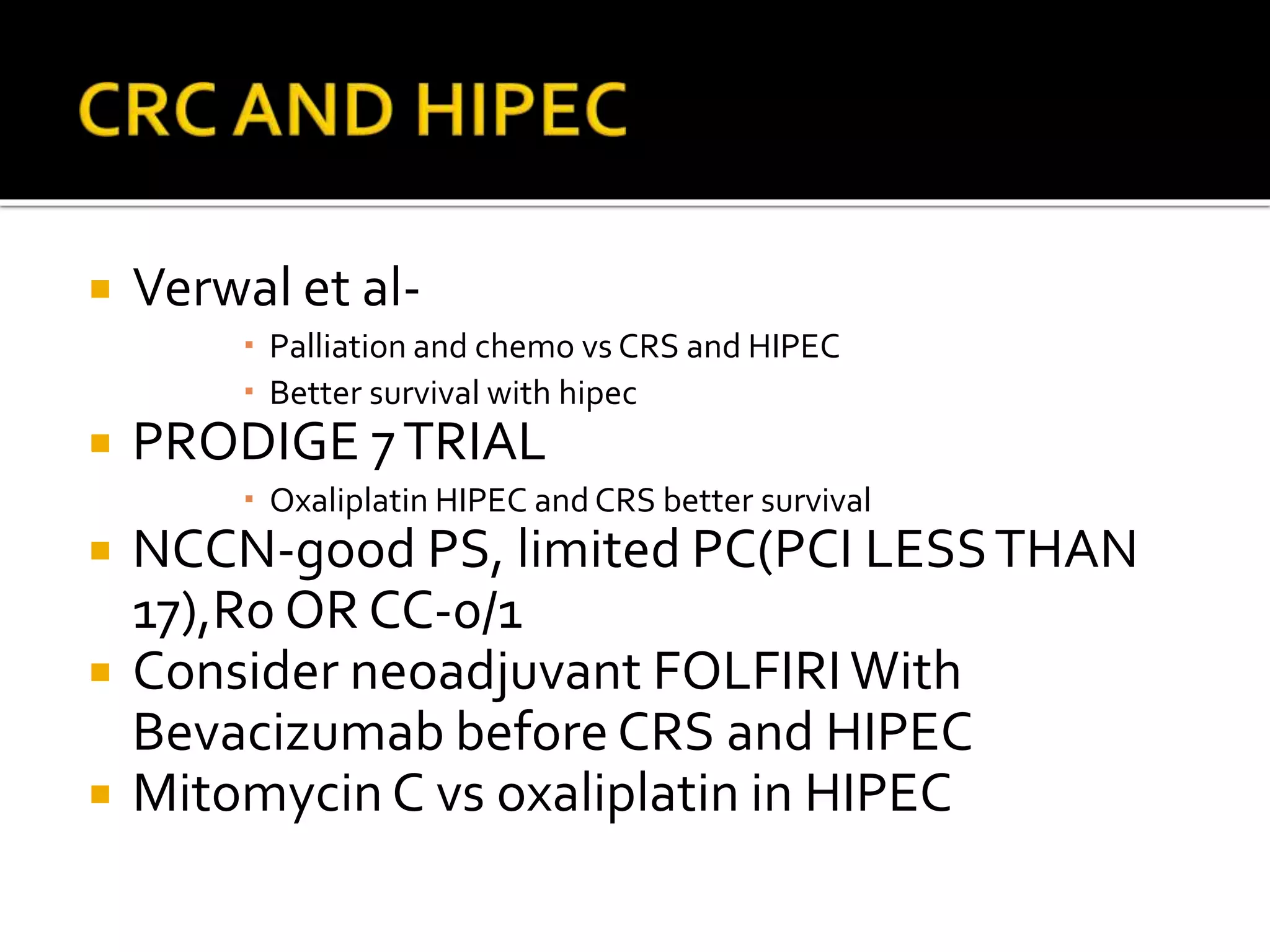
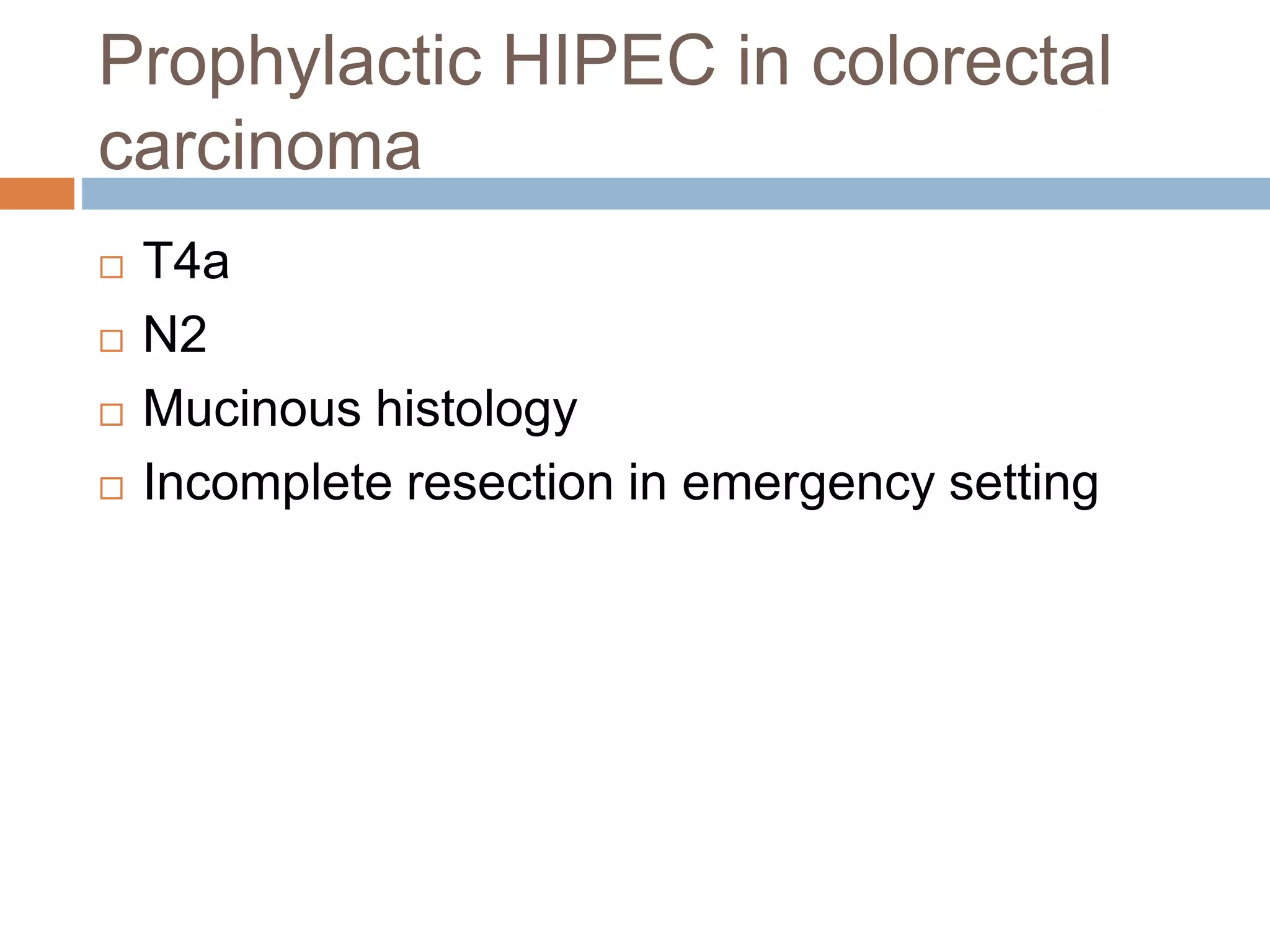
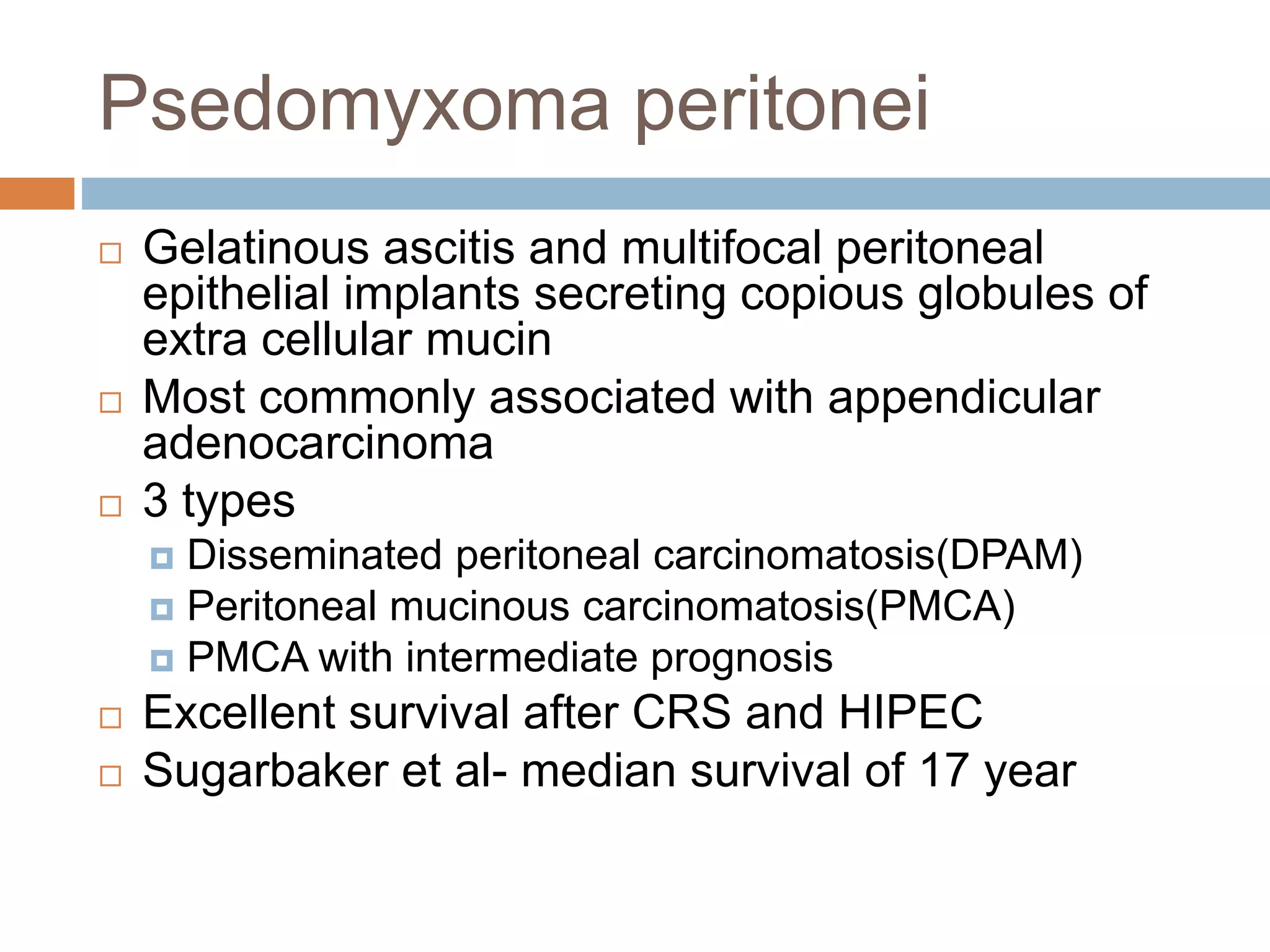
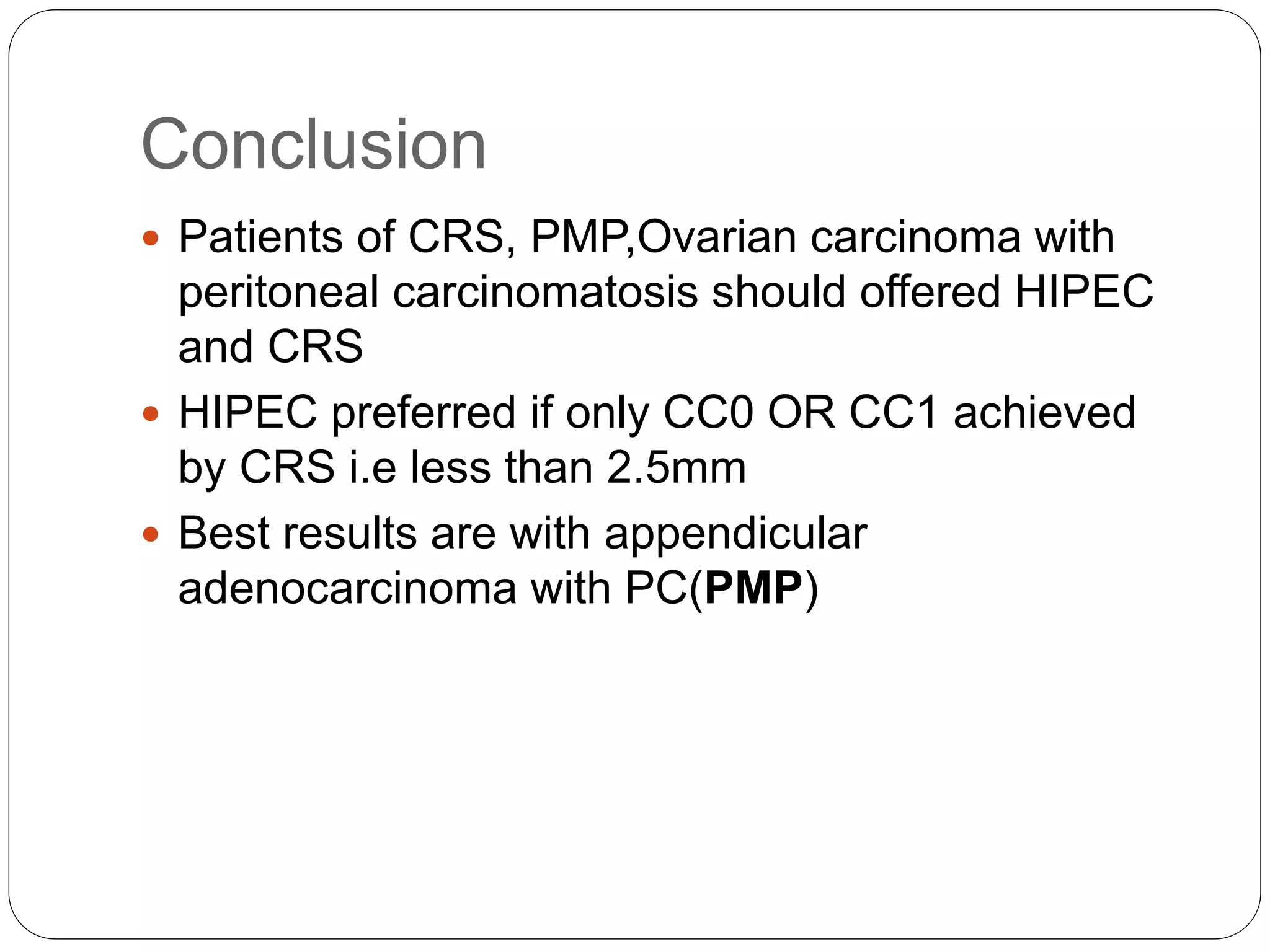
Dr. Yajnadatta Sarangi discusses hyperthermic intraperitoneal chemotherapy (HIPEC), which involves delivering chemotherapy intraperitoneally at high temperatures to treat peritoneal metastasis. HIPEC is usually performed along with cytoreductive surgery to remove all visible tumor deposits. It aims to treat microscopic residual disease remaining after surgery. The document discusses the rationale for HIPEC, techniques, indications, contraindications and complications. It presents evidence that HIPEC combined with cytoreductive surgery improves survival outcomes for several cancer types compared to palliative chemotherapy alone.