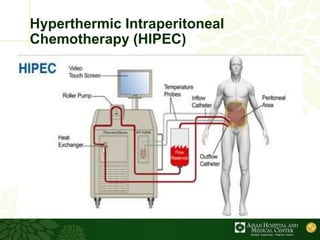
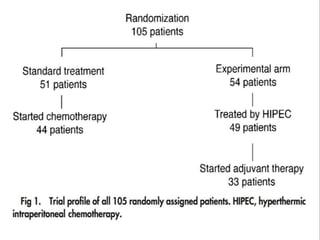
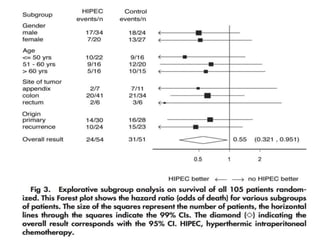
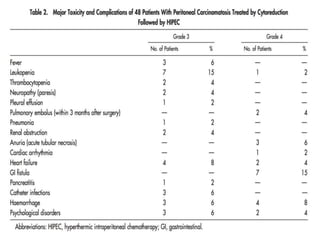
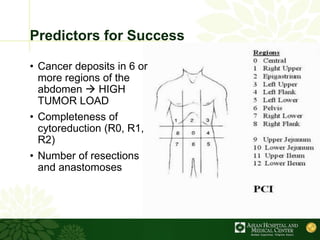
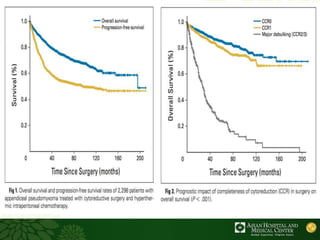
Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) is emerging as a new standard treatment for peritoneal surface malignancies. Traditionally, peritoneal carcinomatosis was considered incurable and treated only with palliative chemotherapy. However, CRS-HIPEC aims to remove all visible tumor deposits surgically and then uses heated chemotherapy in the abdominal cavity to target any remaining microscopic disease. Studies show CRS-HIPEC provides significantly longer survival times compared to intravenous chemotherapy alone, with median overall survivals of 16-36 months. Experts indicate CRS-HIPEC should now be considered the standard of care for select patients with peritoneal metastases from conditions like ovarian