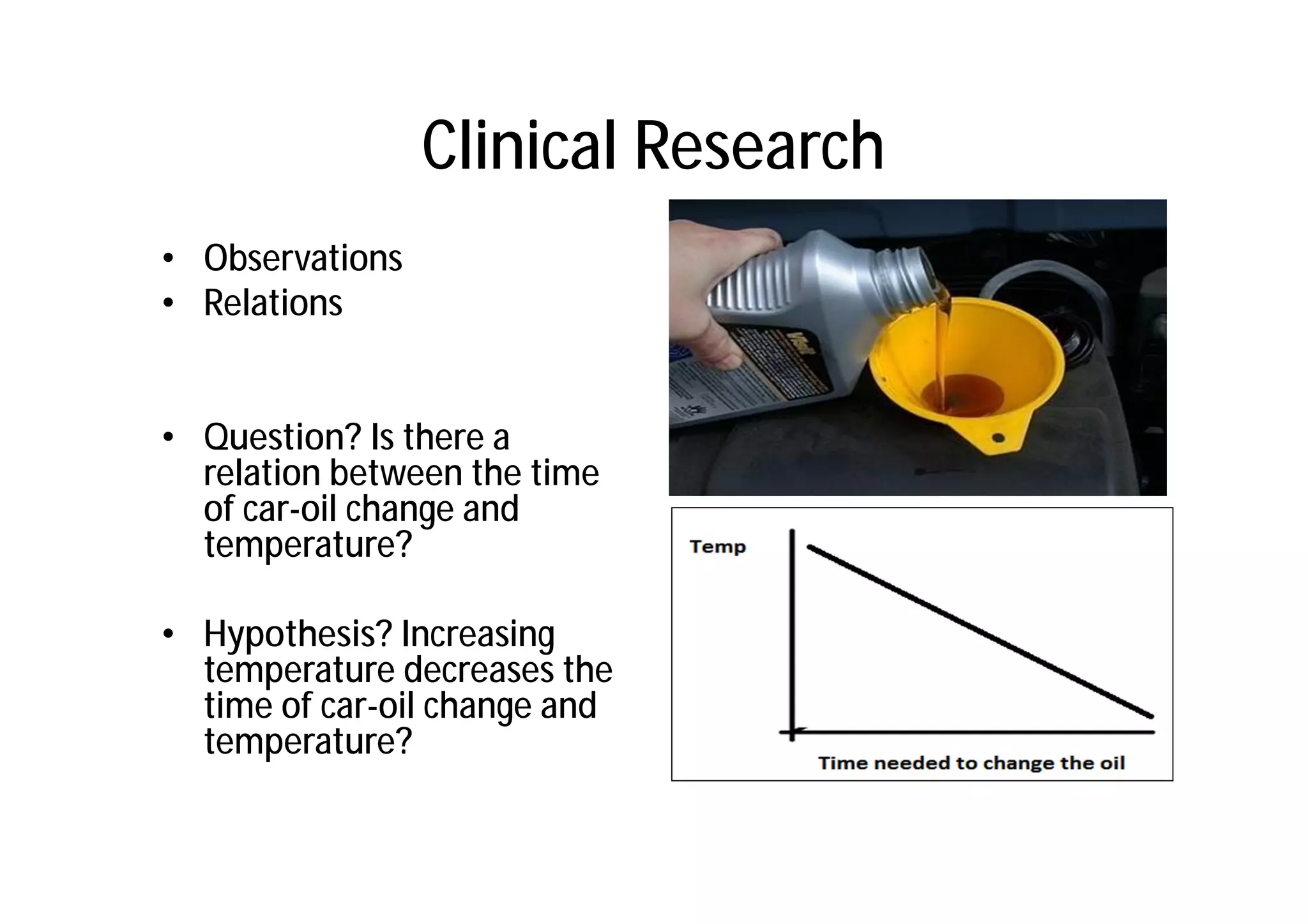
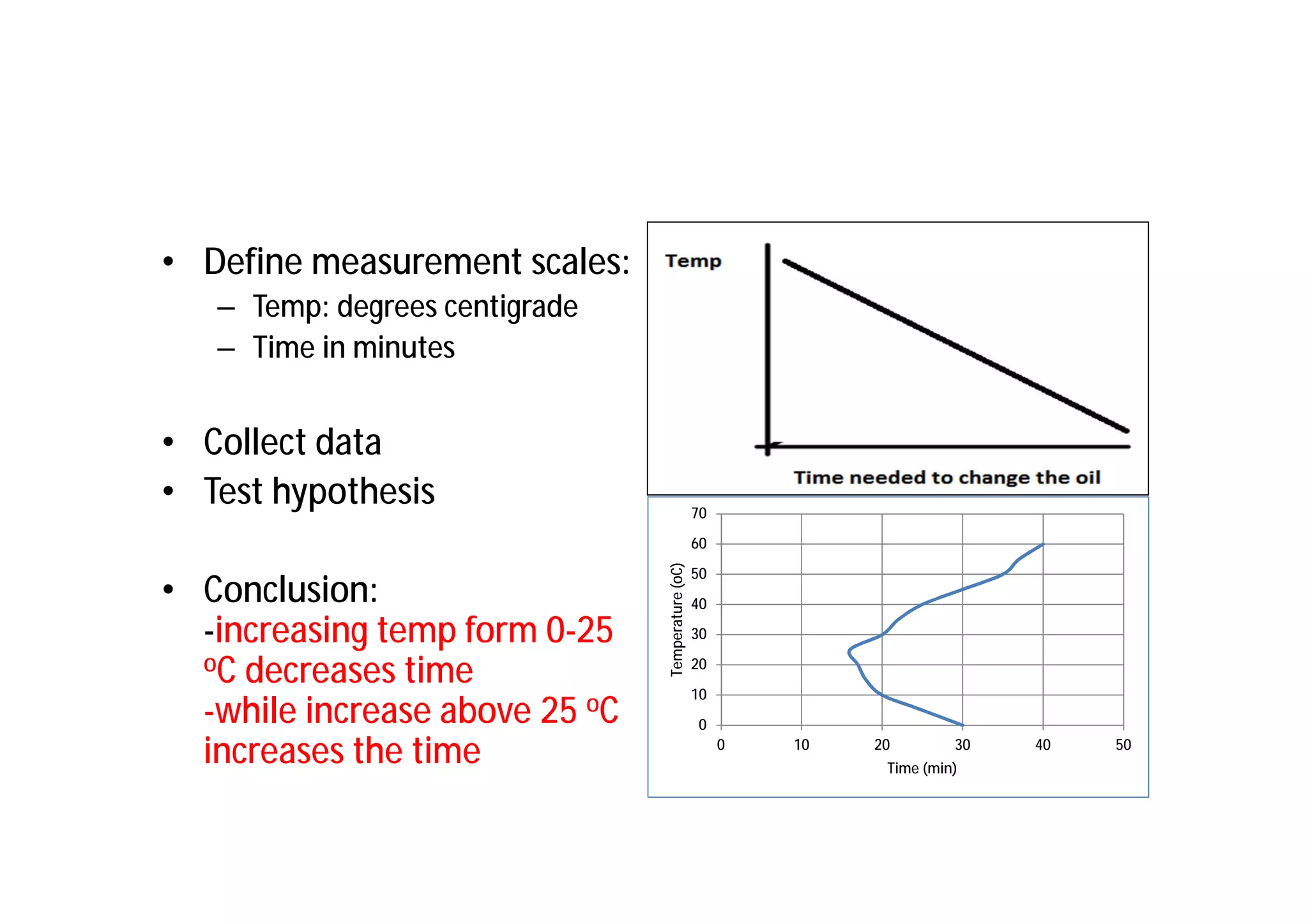
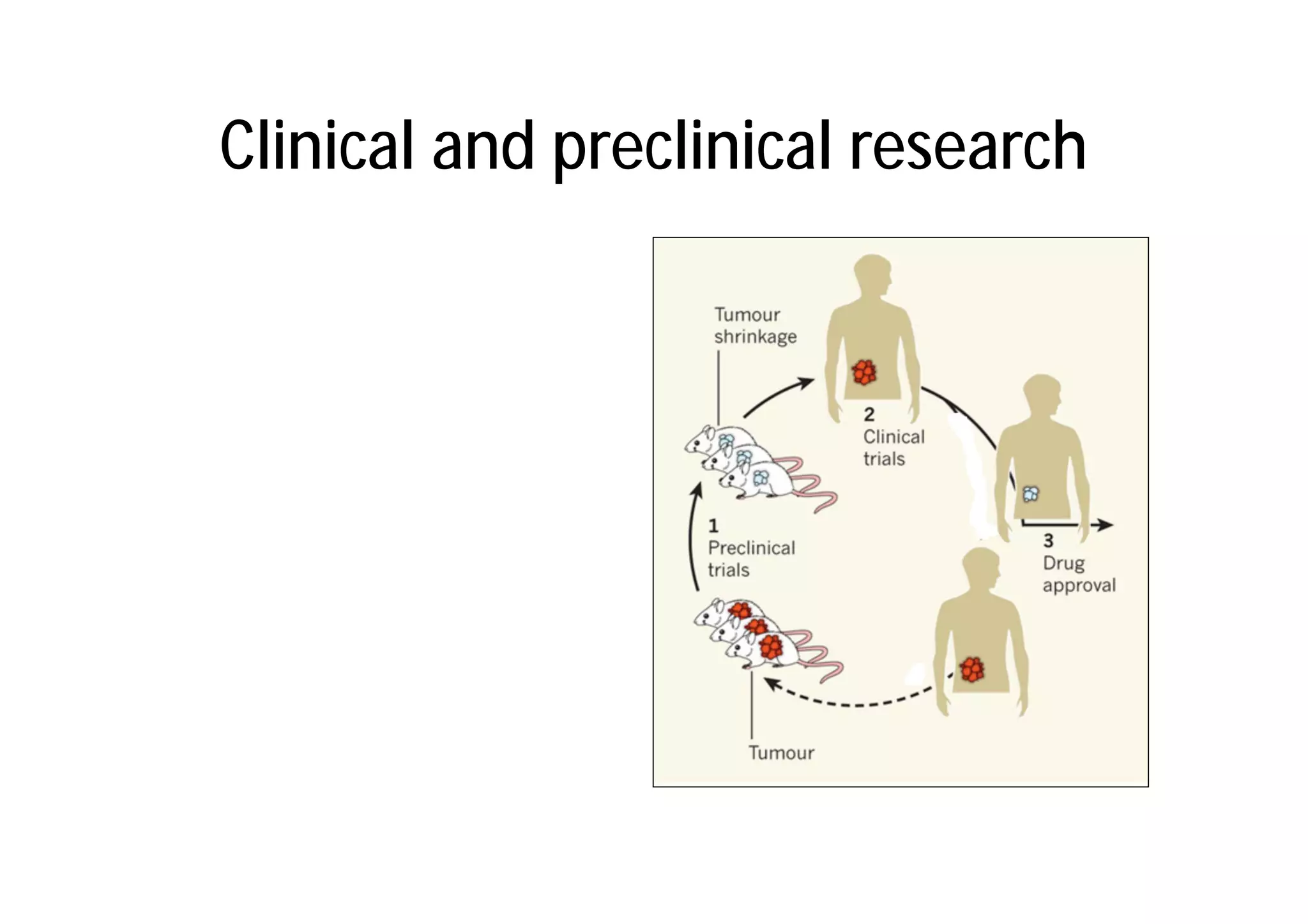
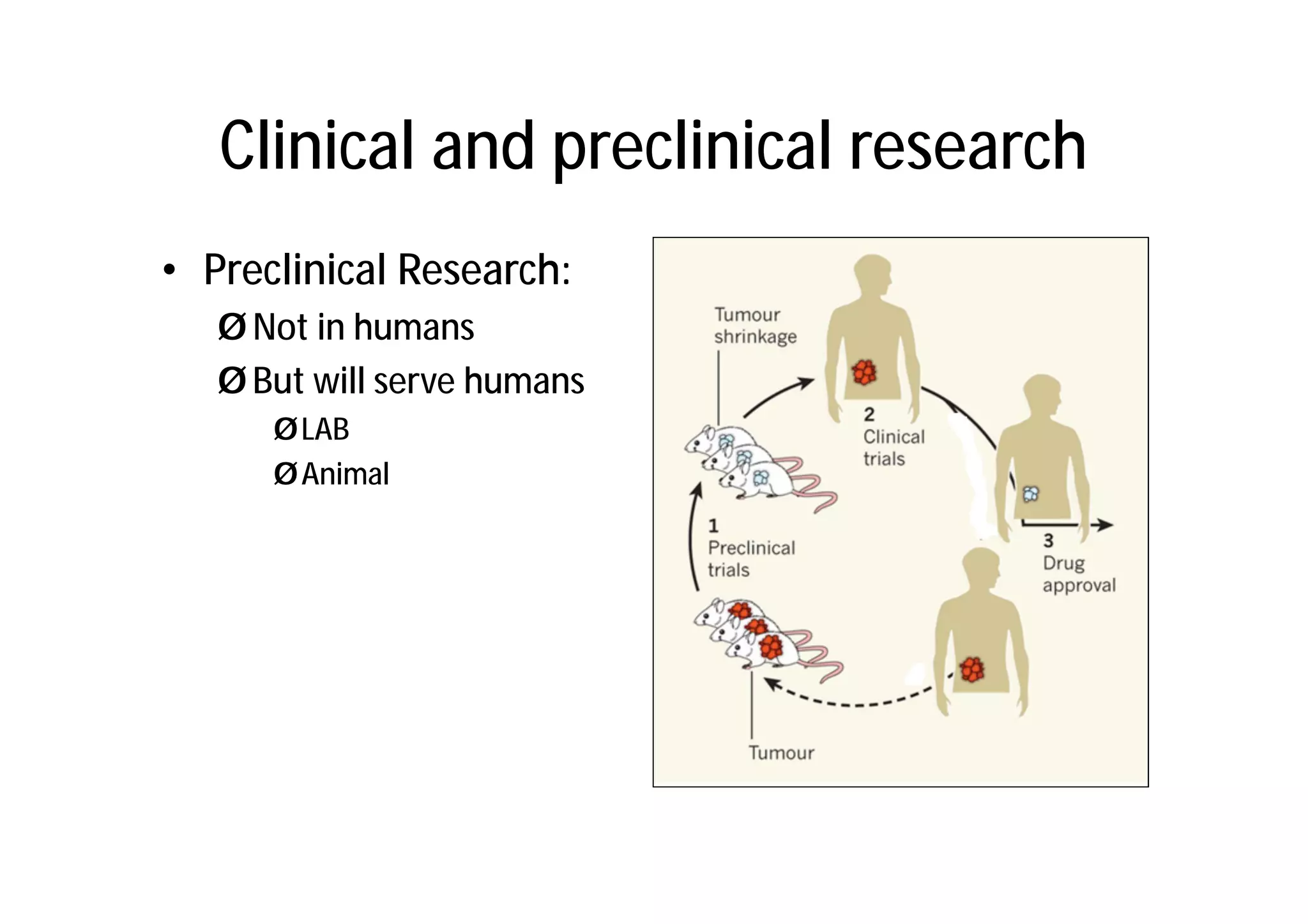
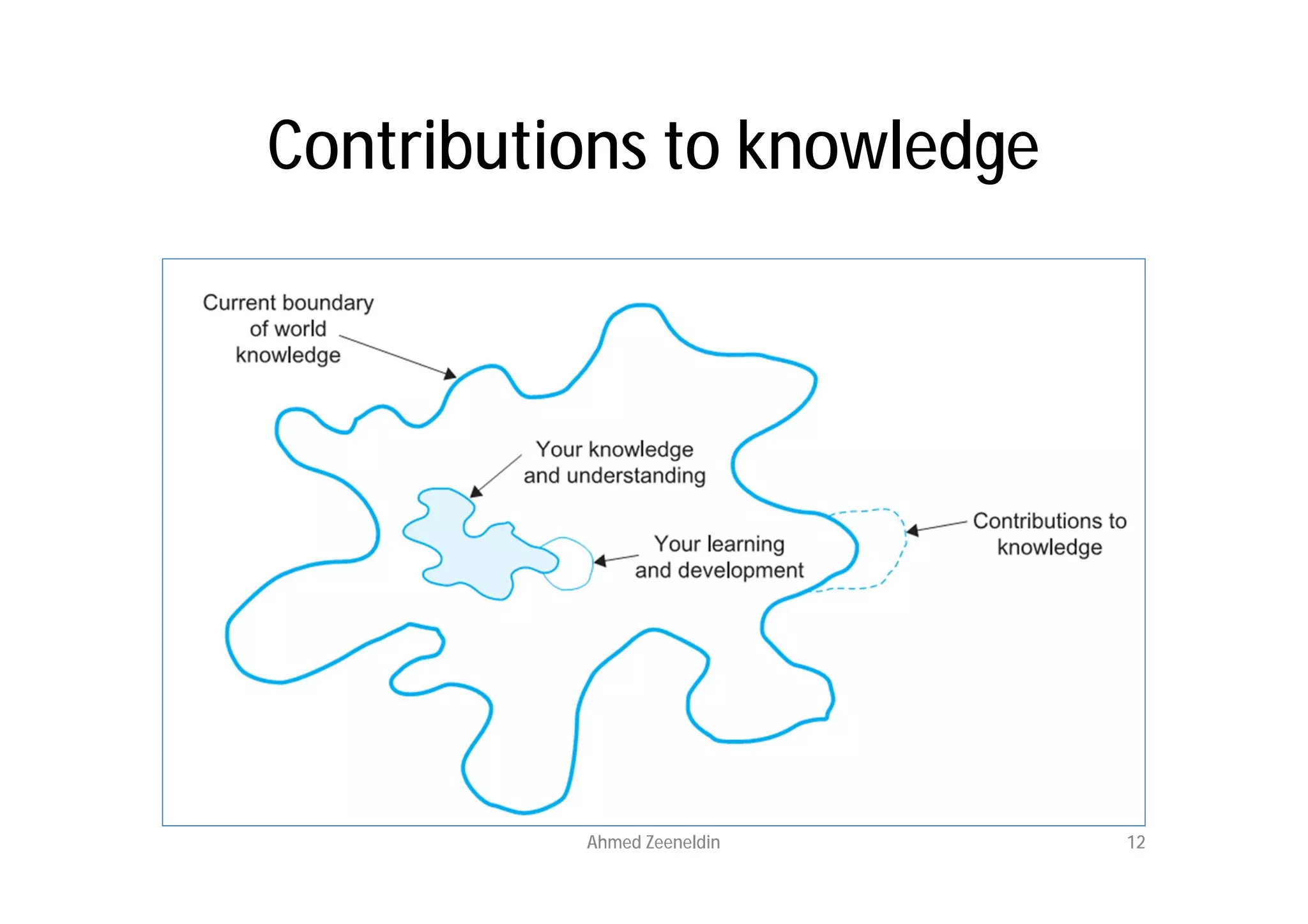
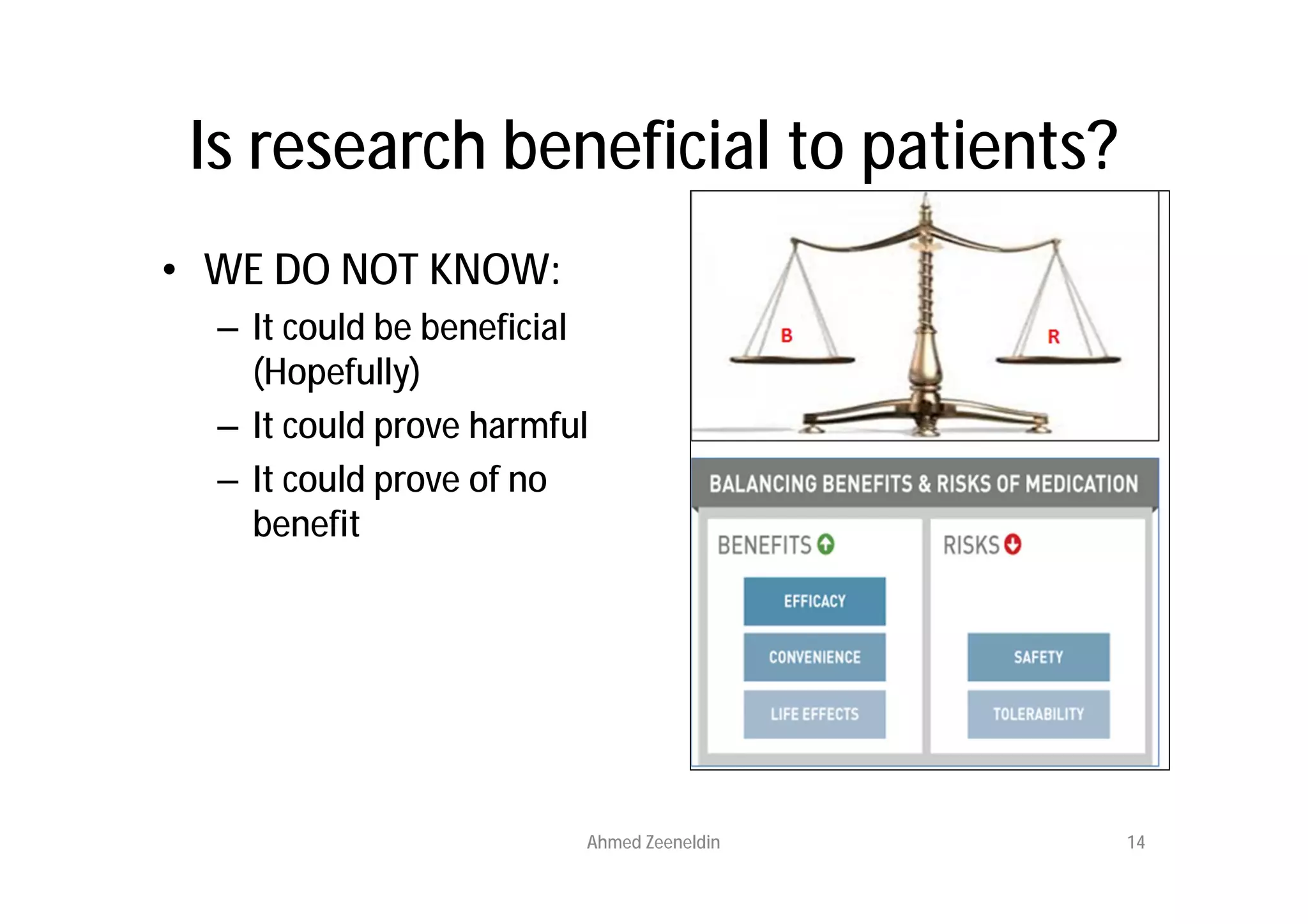
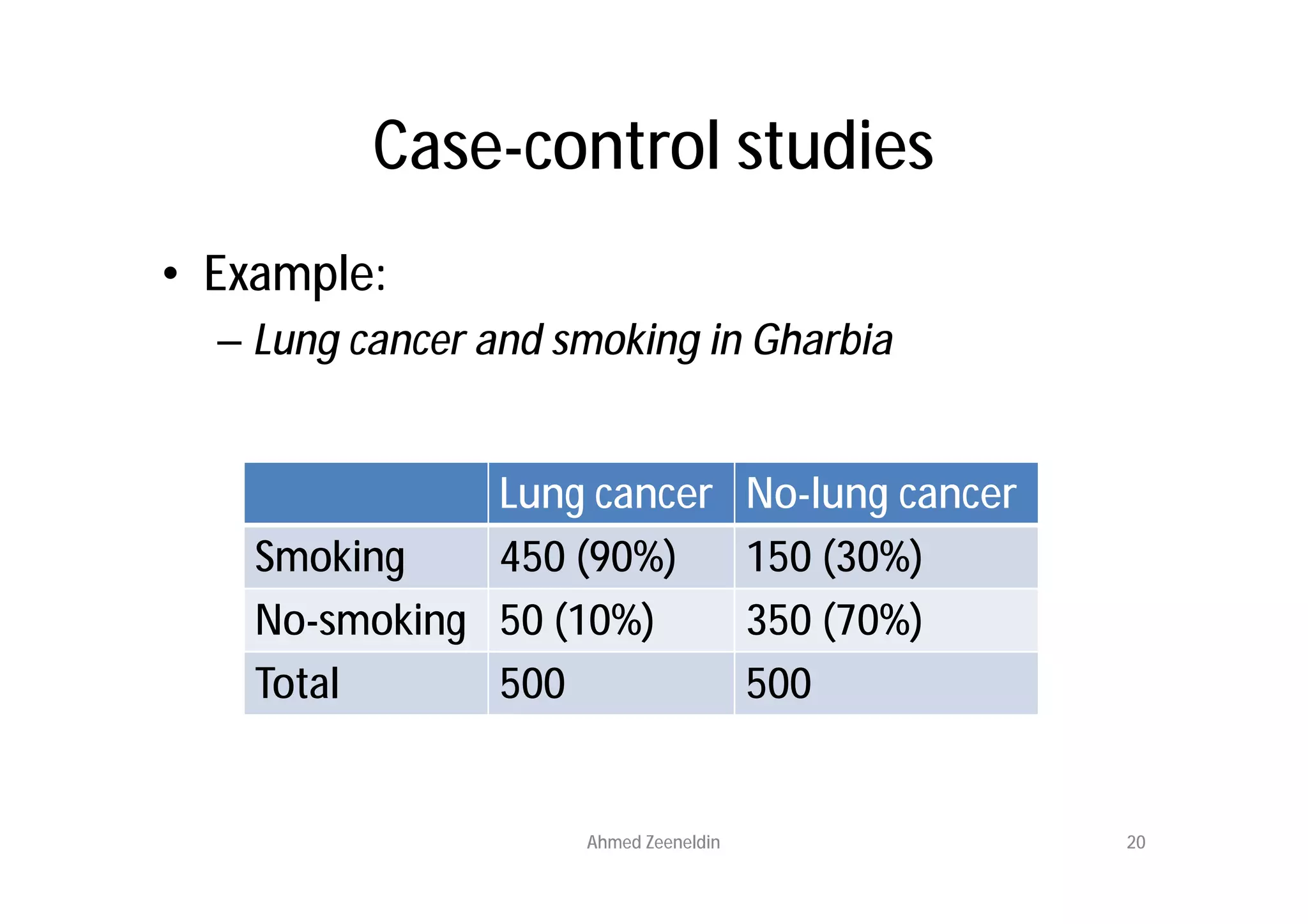
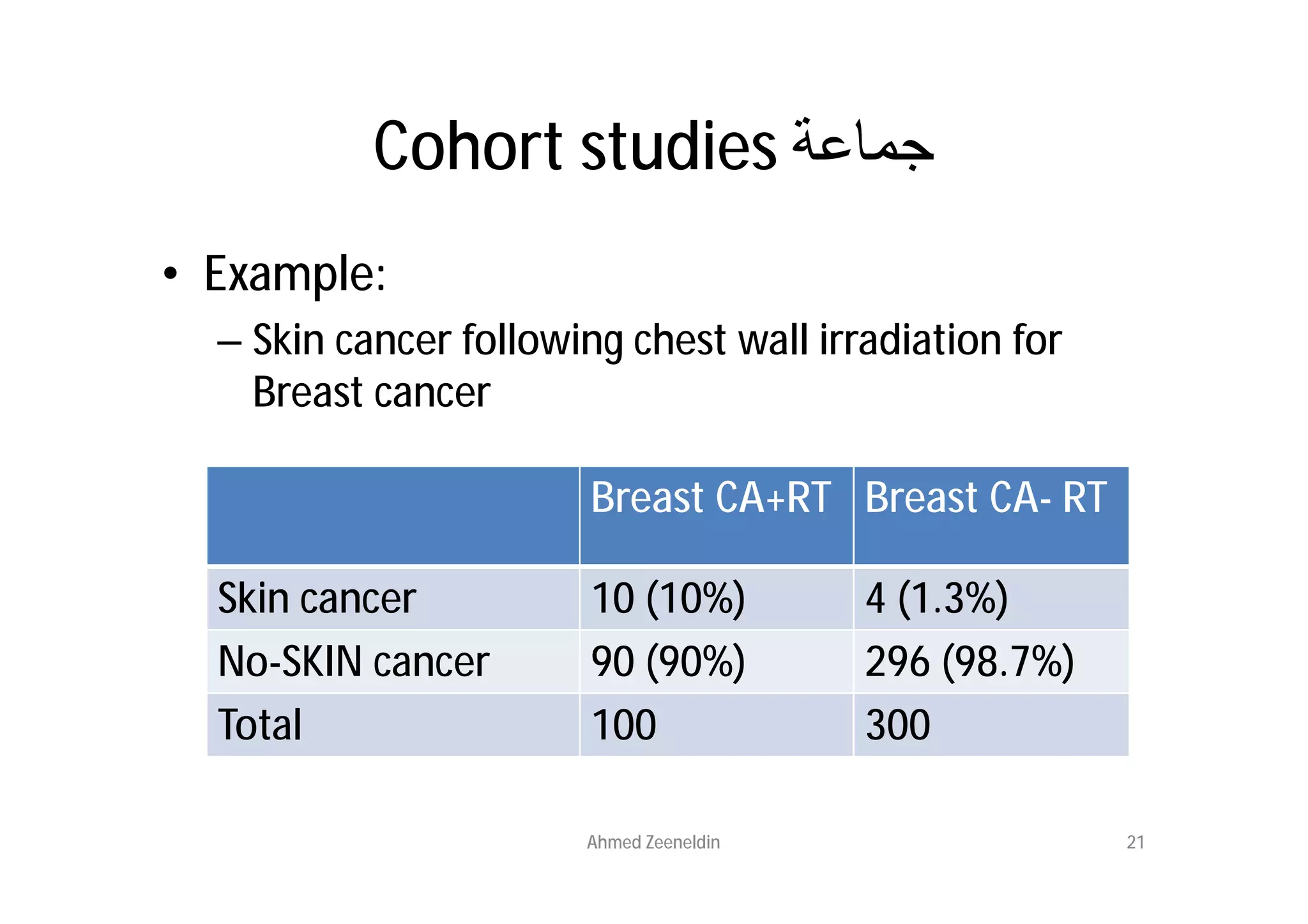
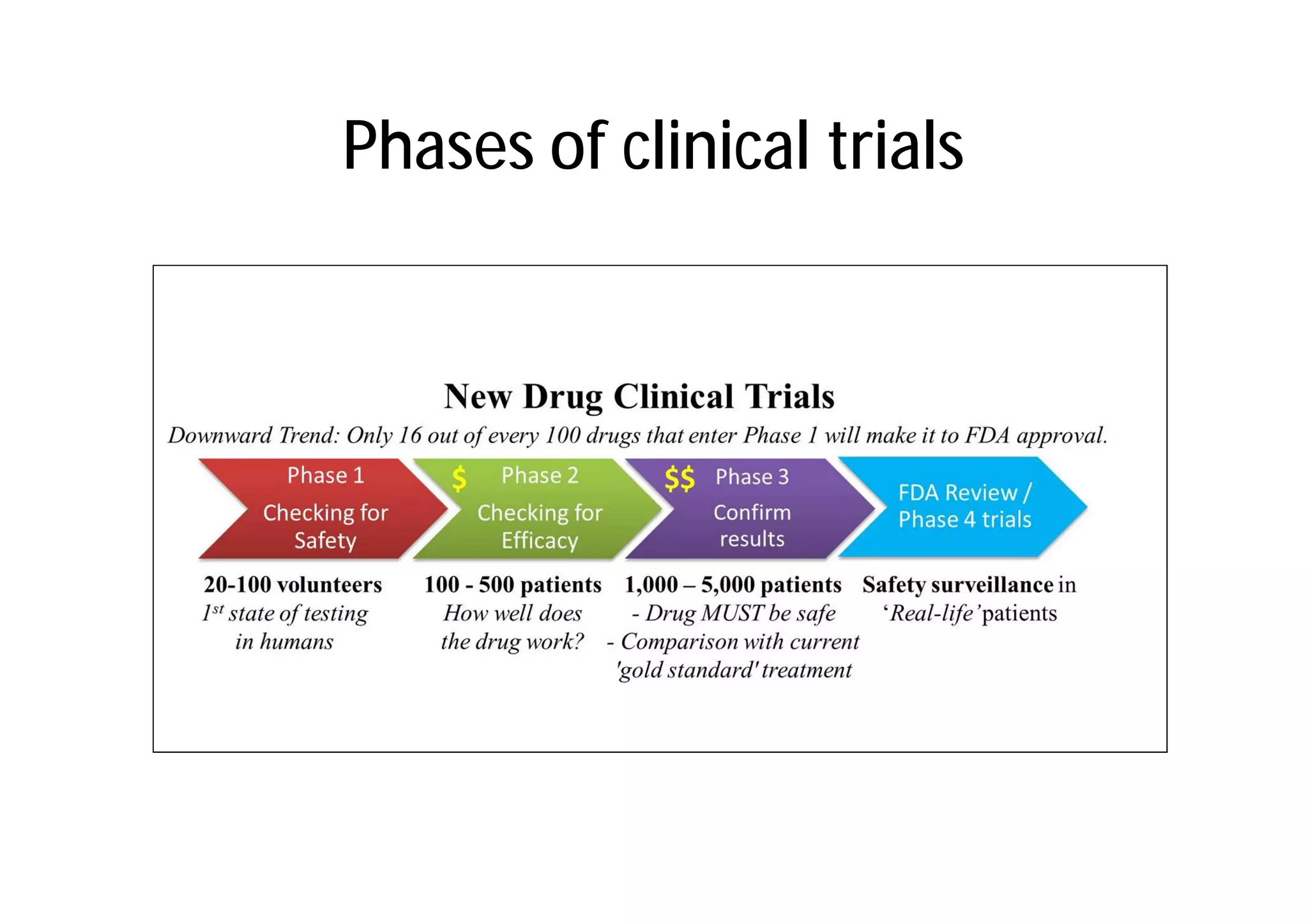
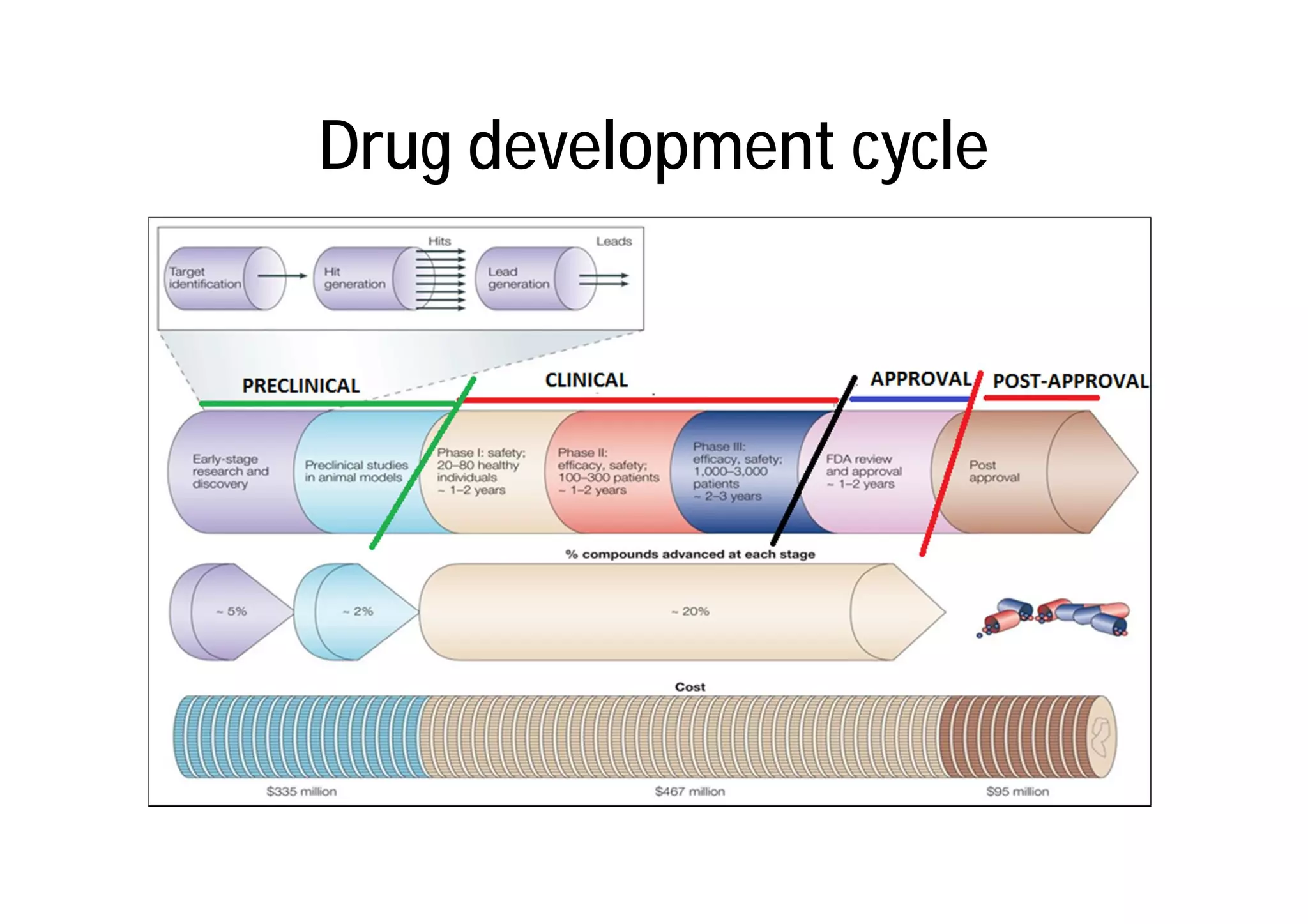
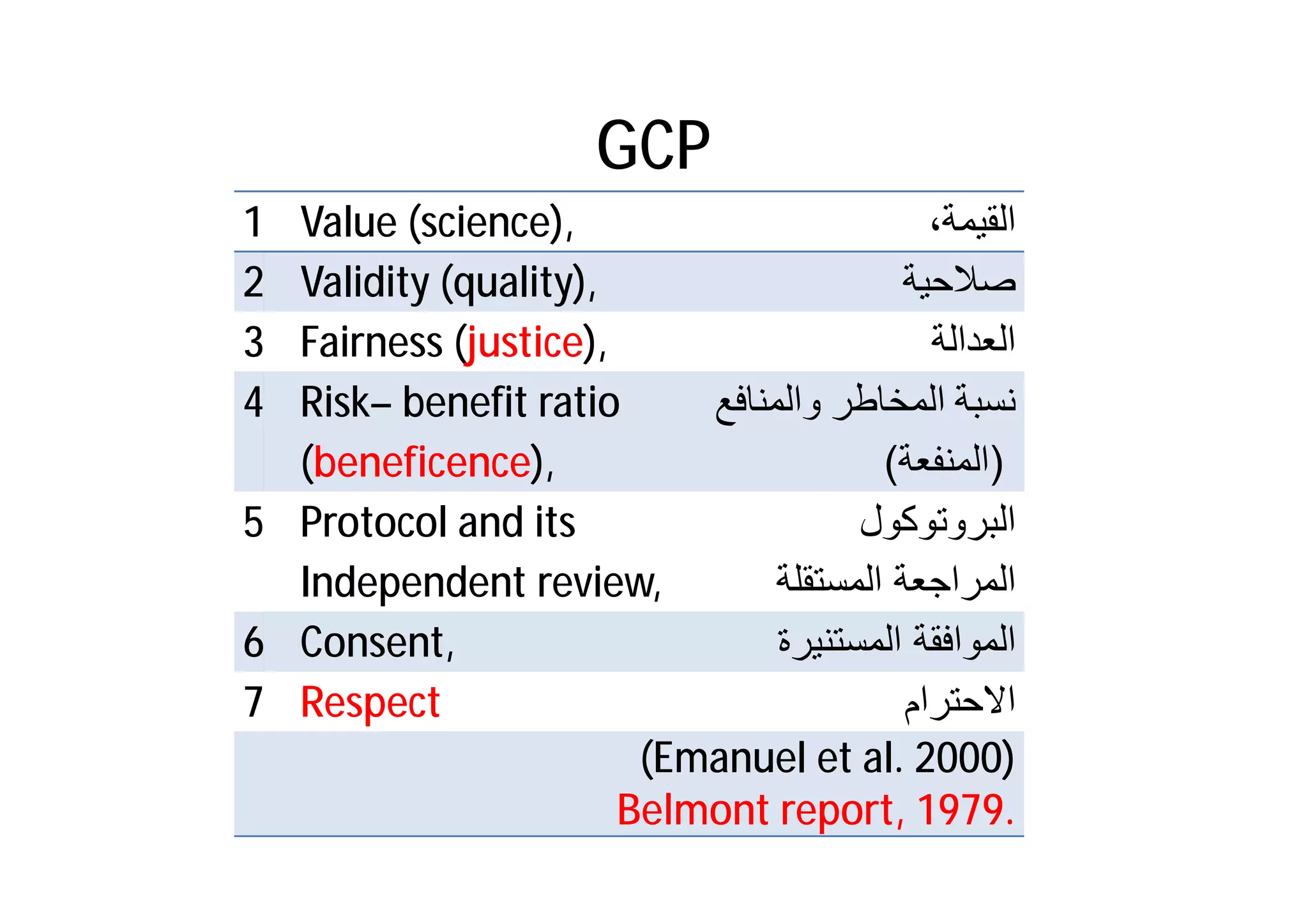
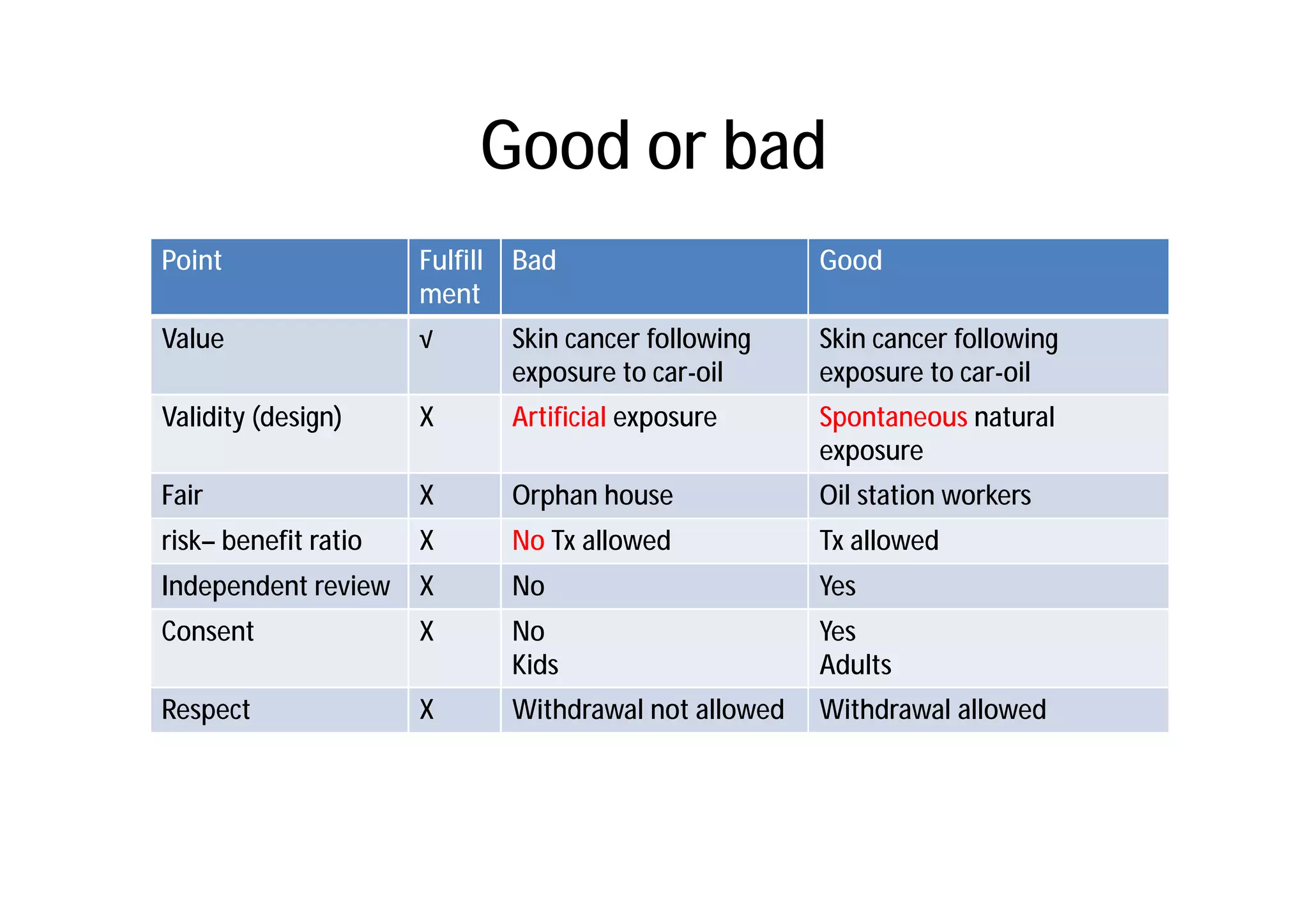
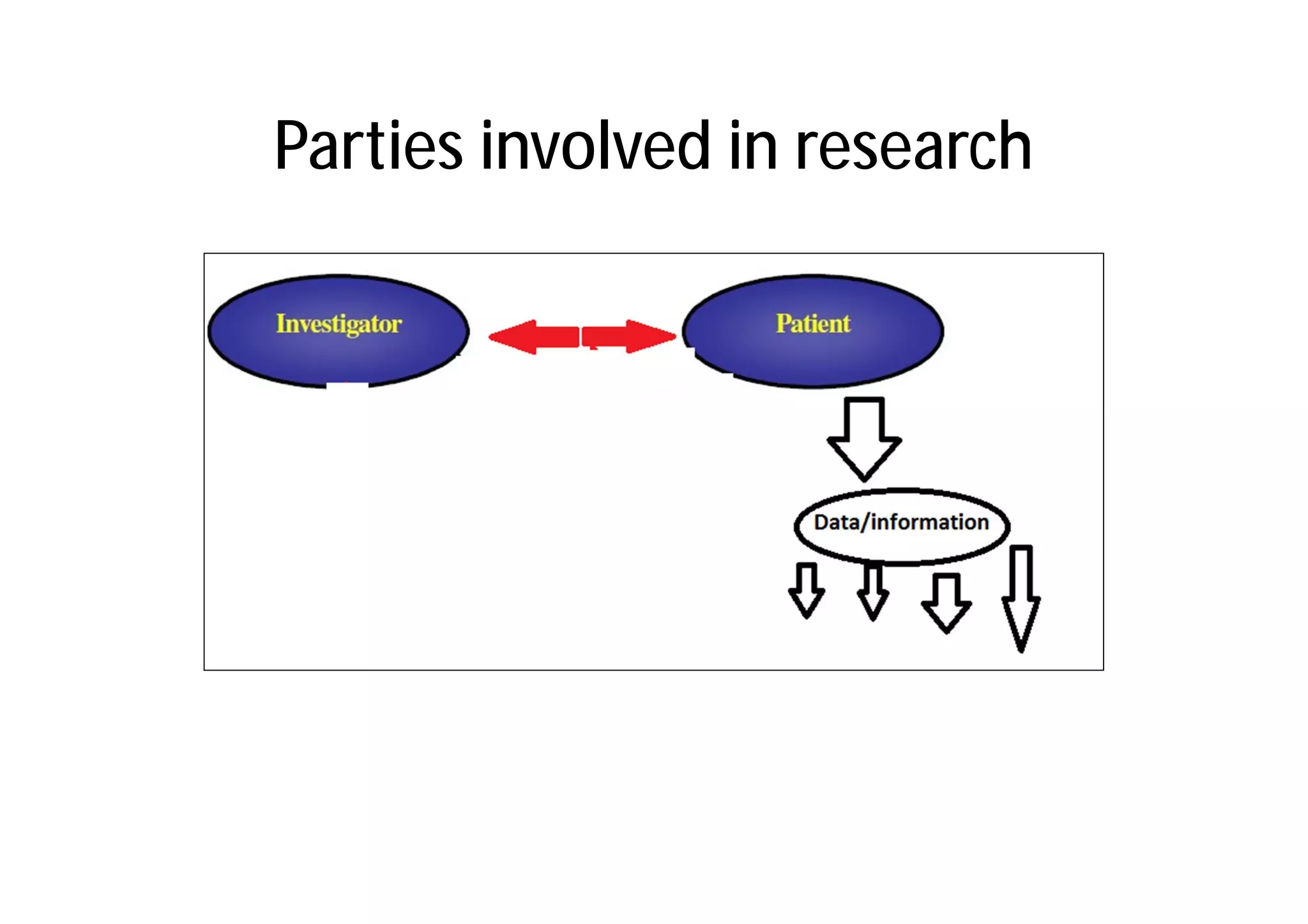
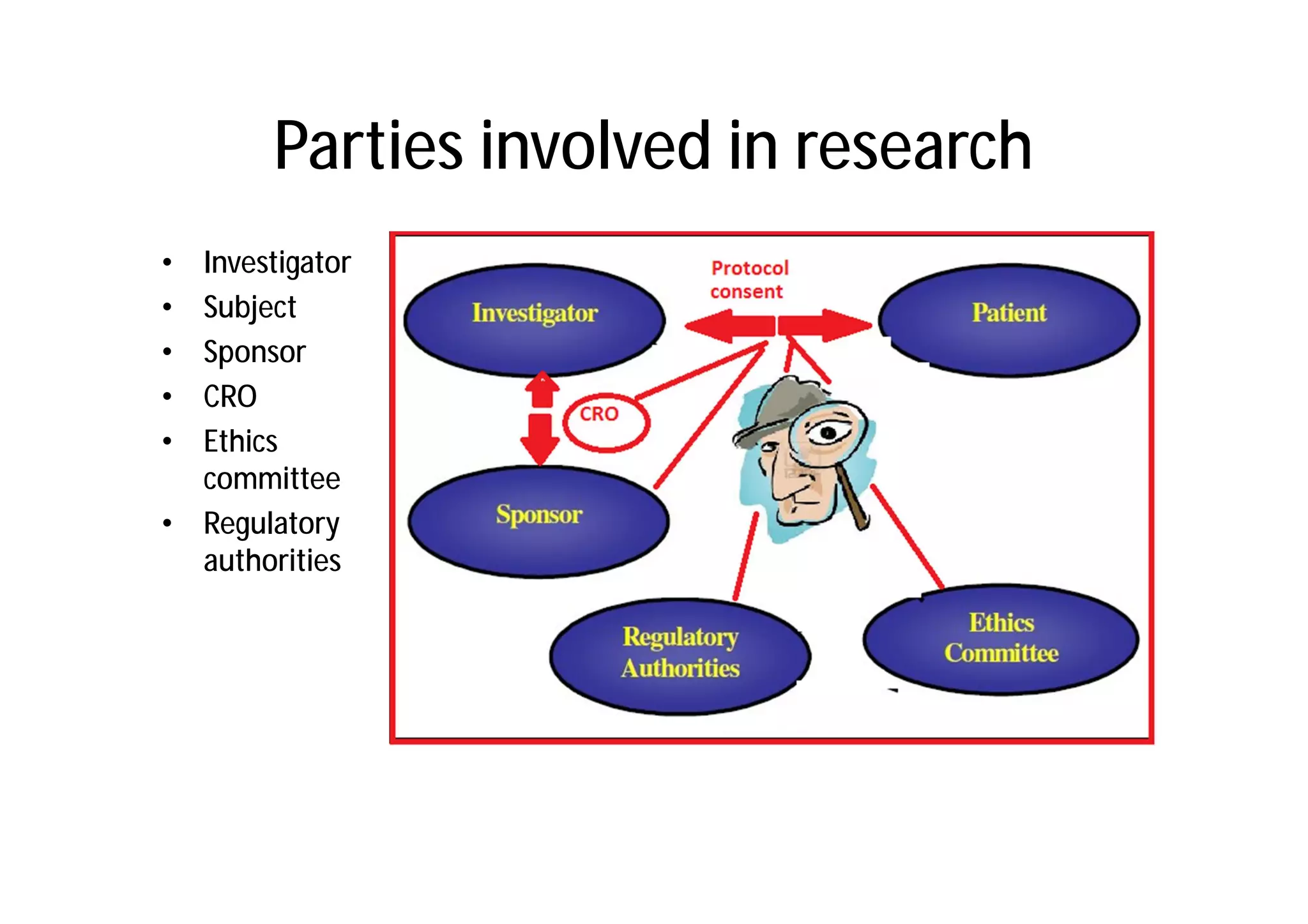
This document provides an introduction to clinical research and good clinical practice (GCP). It discusses the types of scientific and clinical research, including observational and experimental study designs. The document outlines the phases of clinical trials from I to IV. It emphasizes the importance of ethics in research and describes GCP as international ethical and quality standards for clinical research. GCP aims to protect research participants and ensure valid, reliable results. The document lists the parties involved in clinical research, including investigators, sponsors, and ethics committees, and their roles and responsibilities.