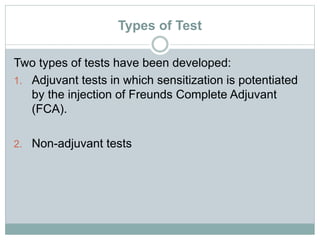
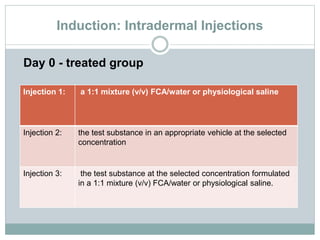
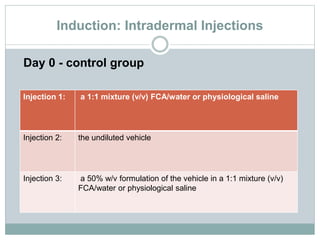
The document summarizes guidelines for skin sensitization toxicity studies using guinea pigs. It describes two common test types - adjuvant tests using Freund's Complete Adjuvant and non-adjuvant tests like the Buehler test. The preferred methods are the Guinea Pig Maximization Test and the non-adjuvant Buehler Test. Both tests involve induction exposure, a rest period, then challenge exposure to determine skin reaction responses. Precise protocols for each test are provided regarding animal selection, housing, dosing, observations, scoring, and reporting of results.