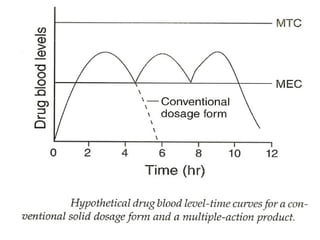
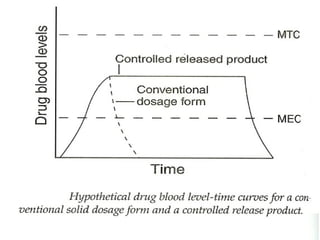
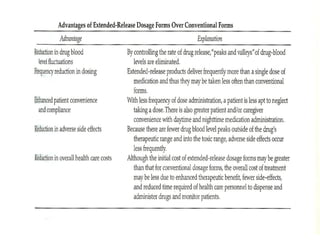
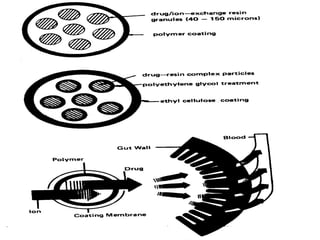
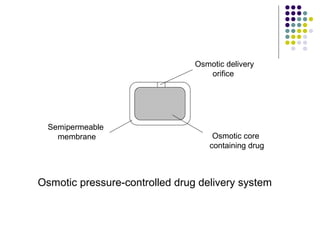
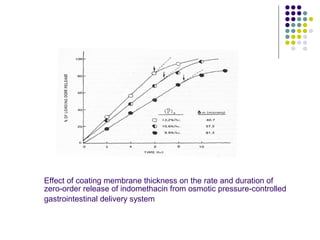
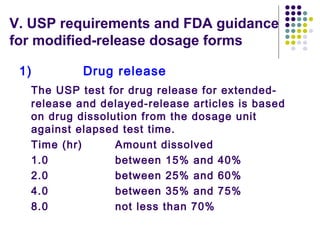
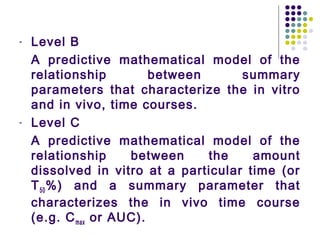
This document discusses modified-release oral dosage forms, including extended-release and delayed-release forms. It describes various technologies used to modify drug release rates, such as coatings, matrices, and osmotic pumps. It also covers terminology, drug candidates suited for modified dosing, clinical considerations, and FDA/USP regulations regarding testing, labeling and in vitro-in vivo correlations. The goal of these dosage forms is to reduce dosing frequency while maintaining therapeutic drug levels over time.