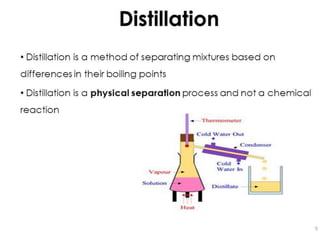
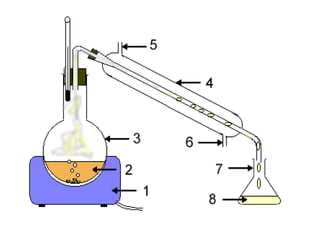
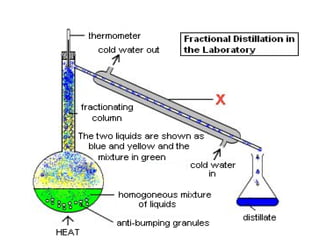
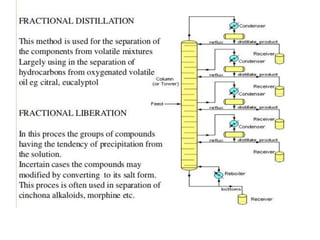
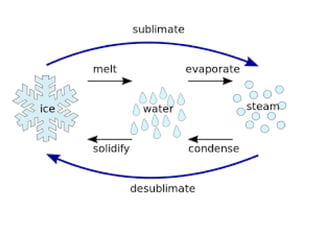
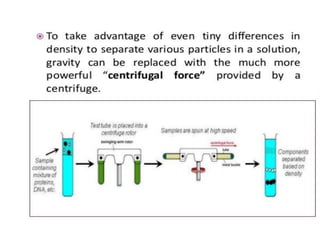
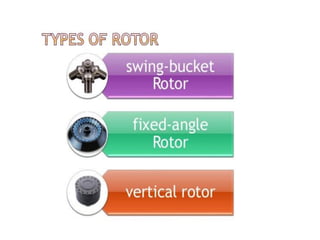
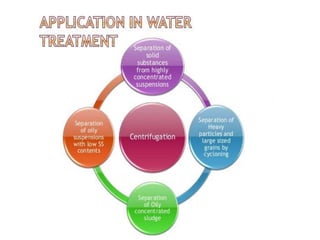
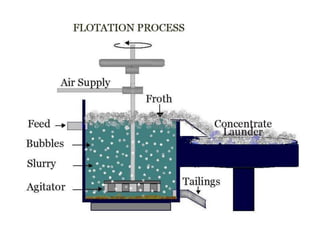
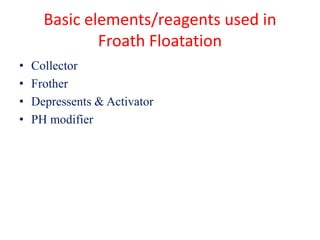
Fractional distillation and sublimation are two non-chromatographic separation techniques. Fractional distillation uses a fractional column to separate liquids with similar boiling points, such as ethanol and water. Sublimation transitions substances directly from solid to gas without an intermediate liquid phase, as seen with dry ice. Derivatization is a chemical reaction that converts polar functional groups to nonpolar groups to make molecules volatile for analysis by gas chromatography. Froth flotation selectively separates hydrophobic and hydrophilic materials using bubbles to carry hydrophobic particles to the surface in a stable froth.