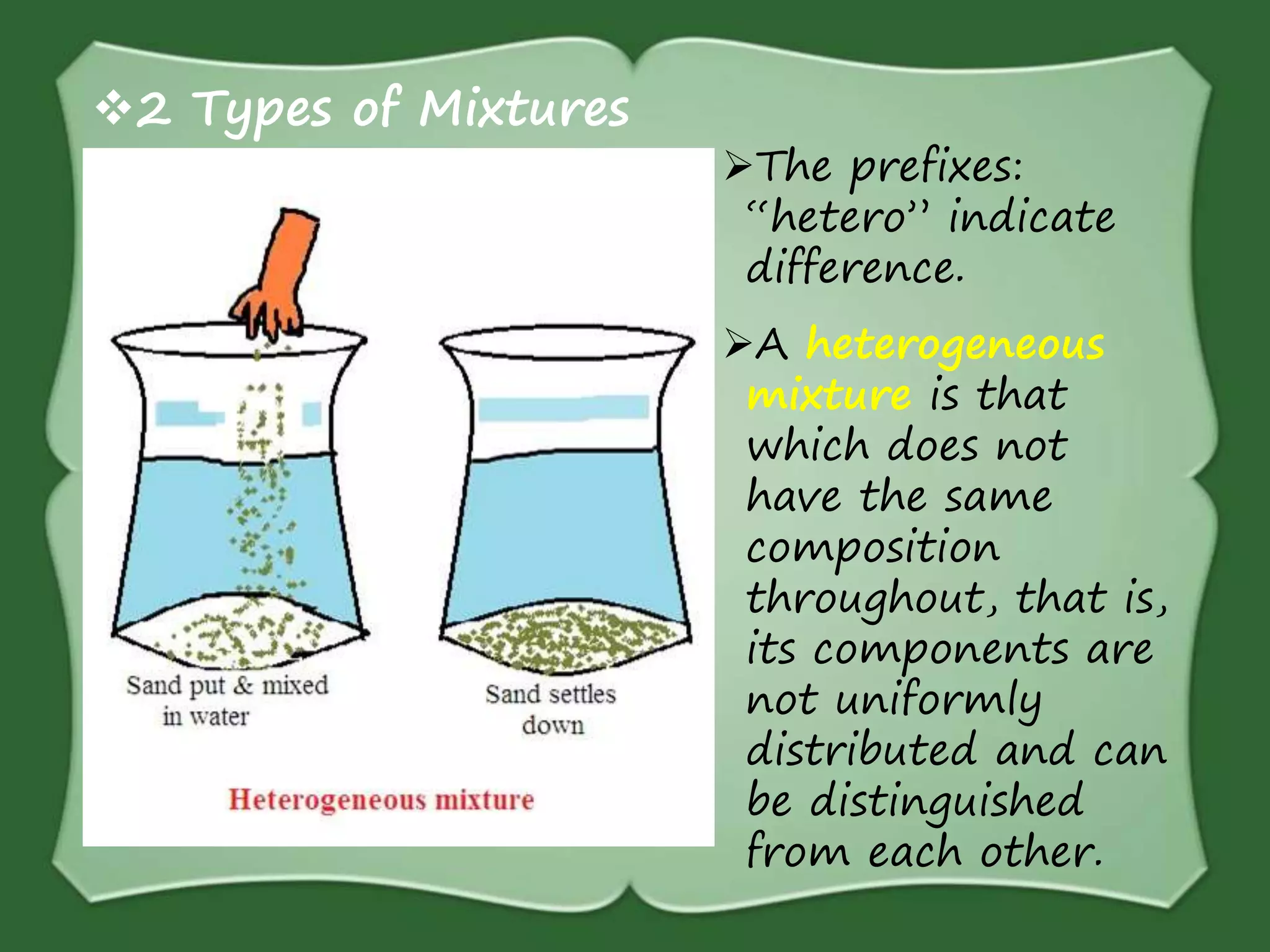
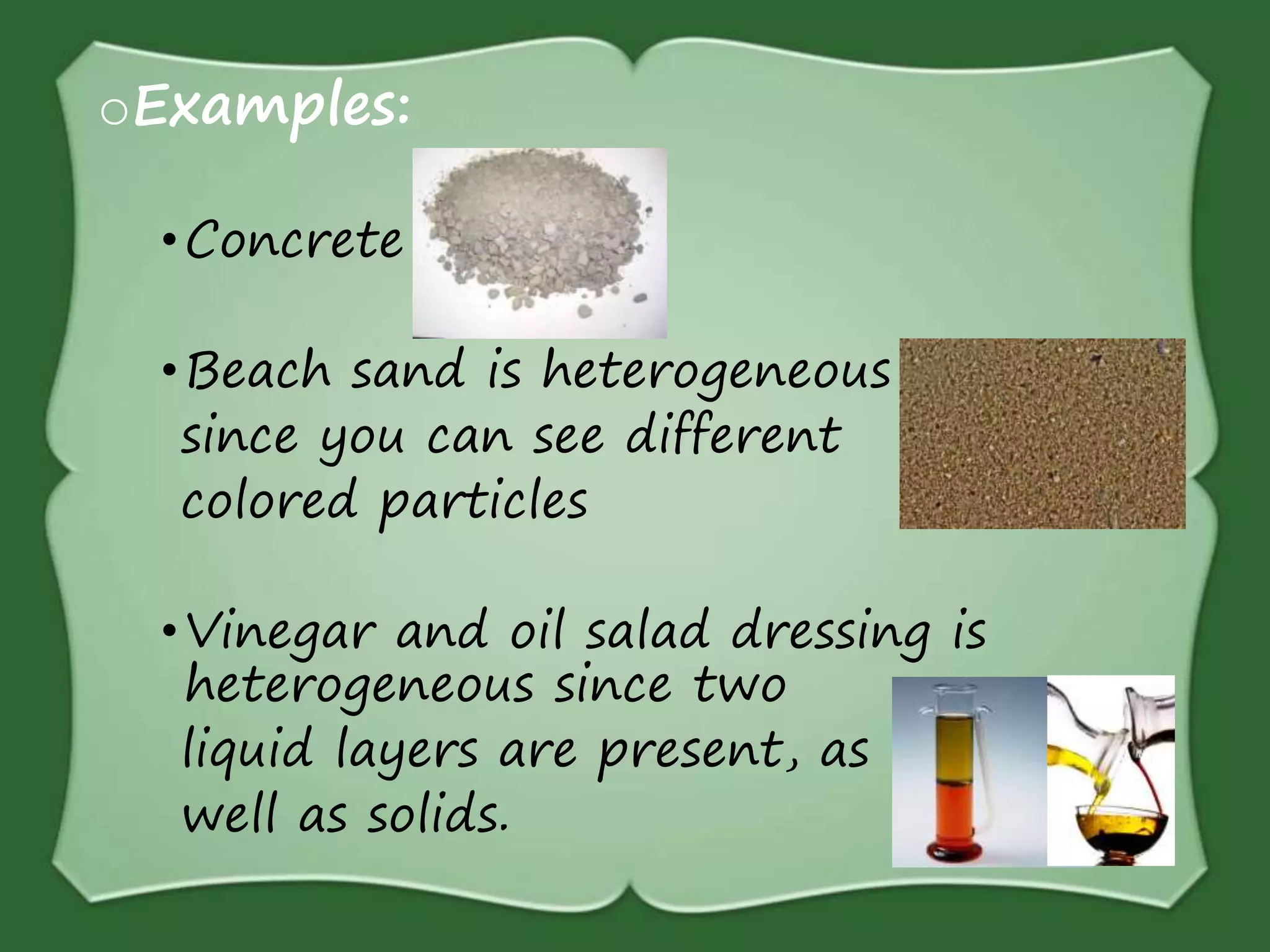
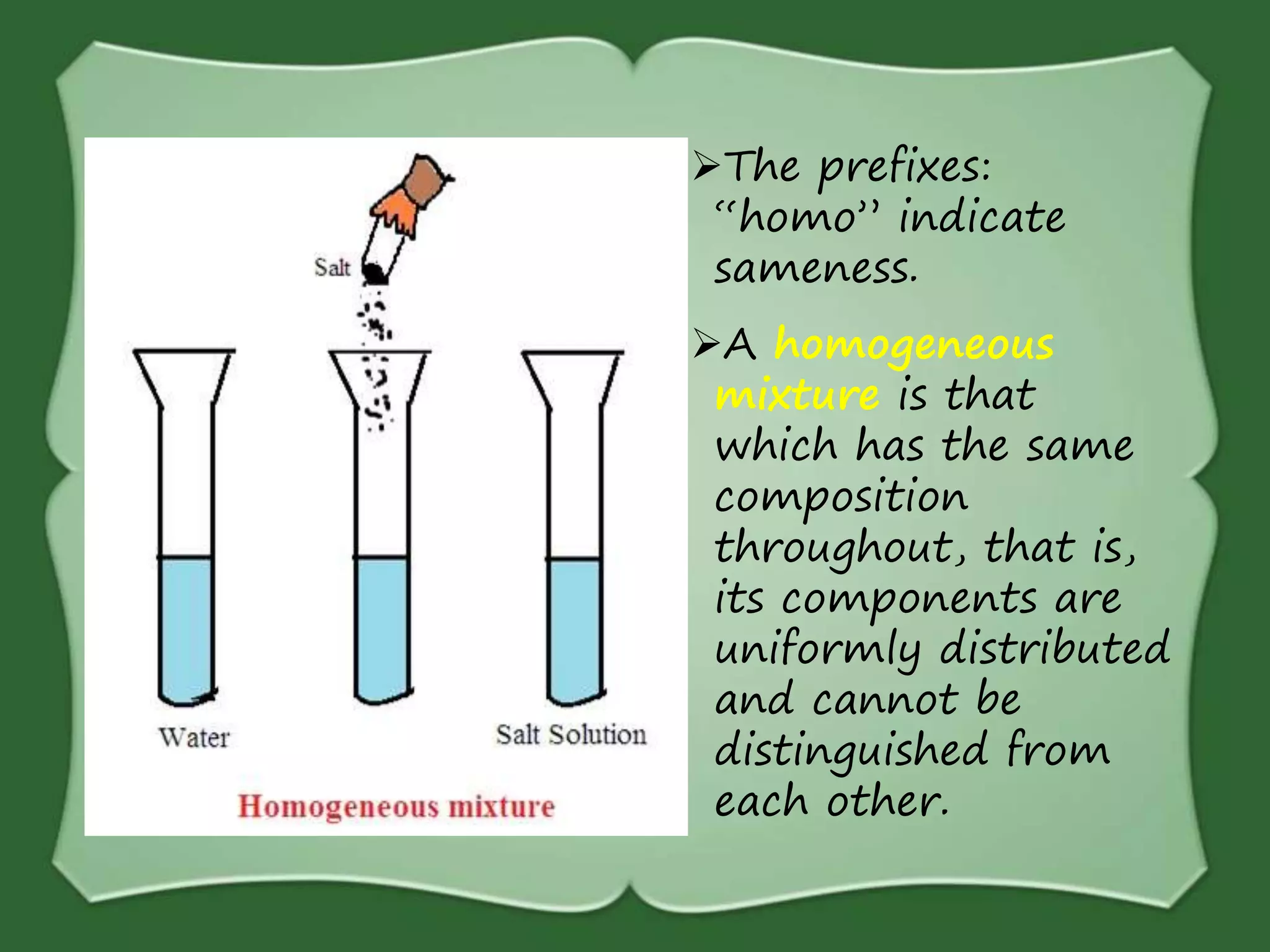
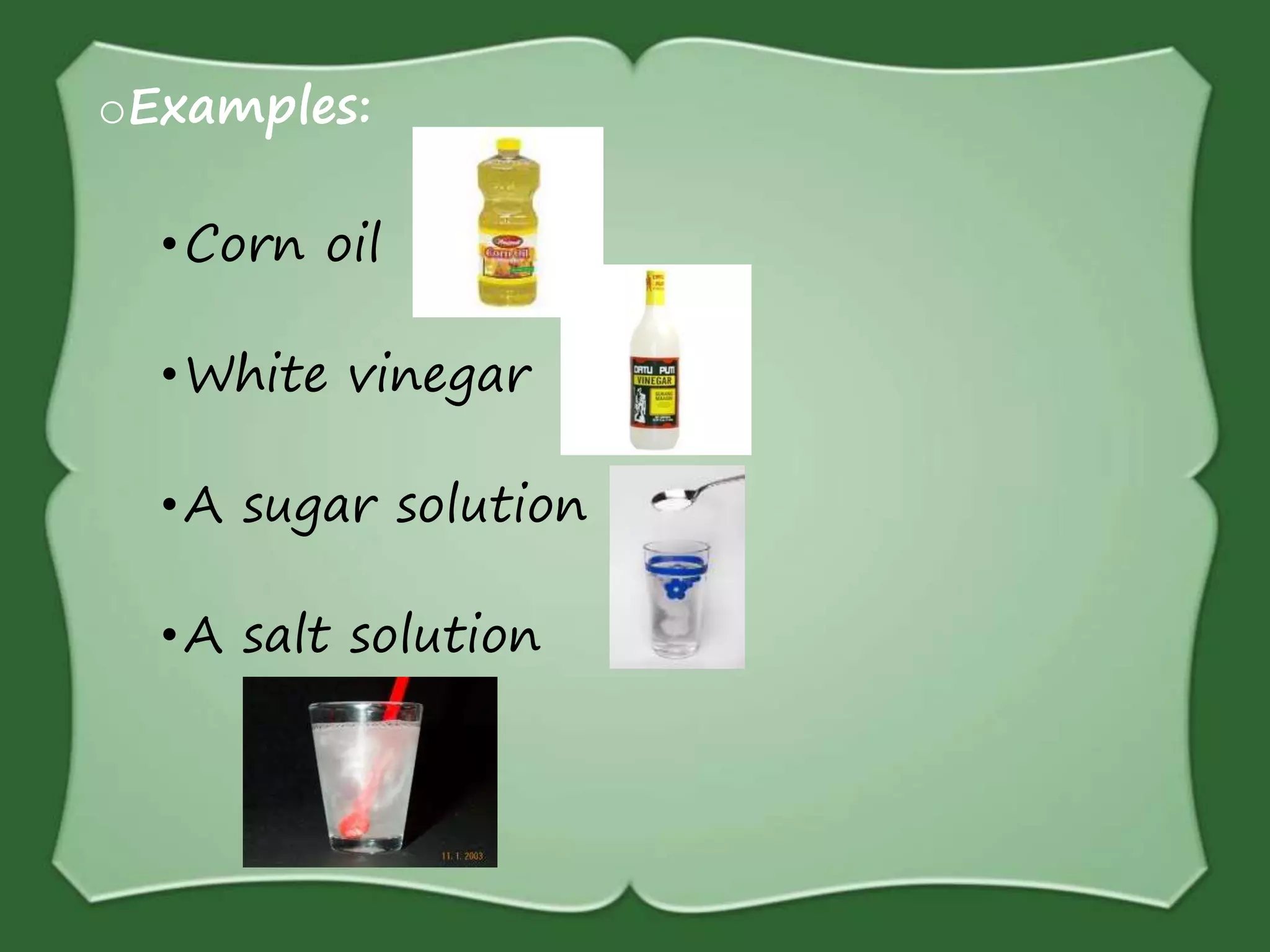
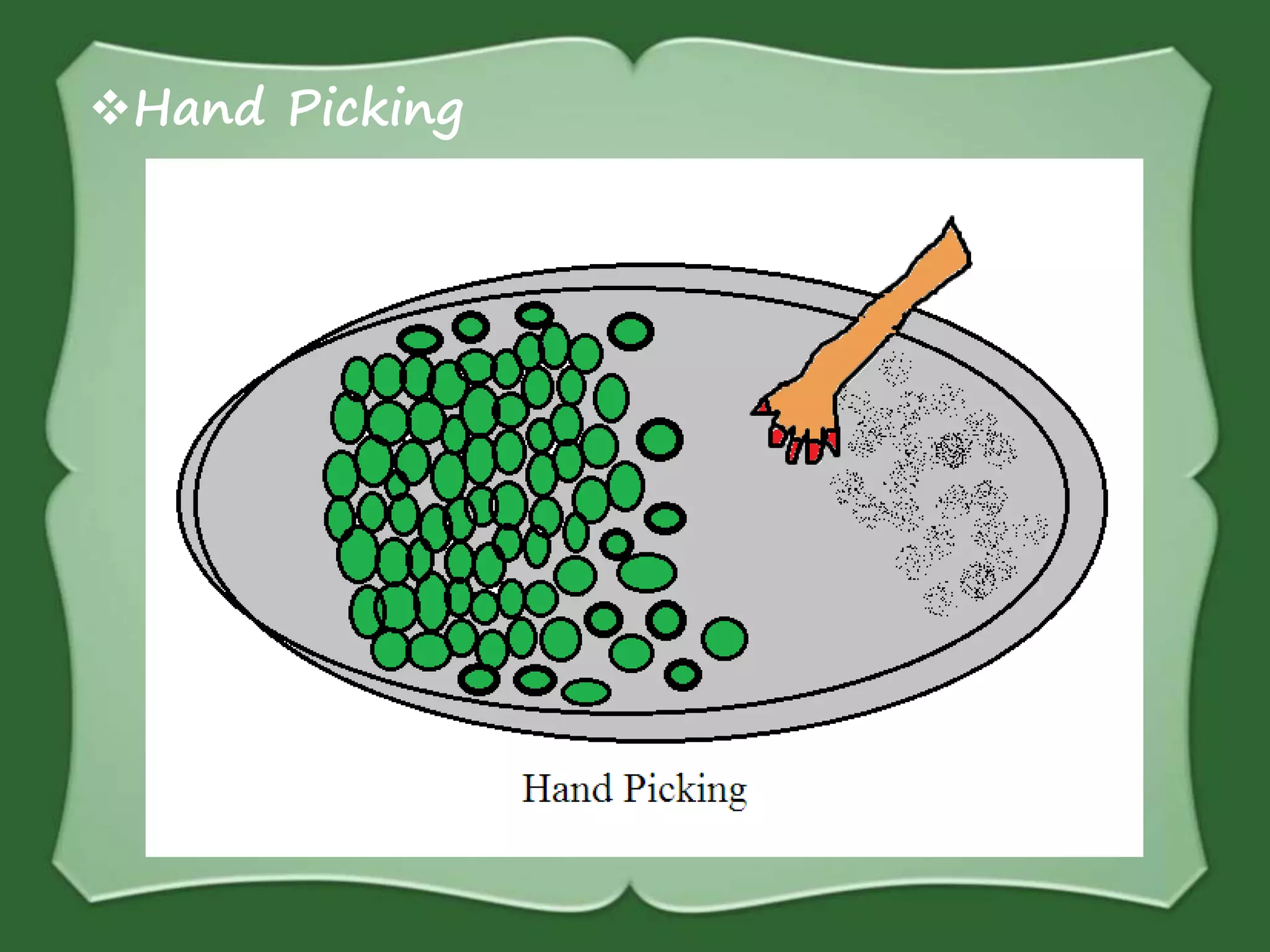
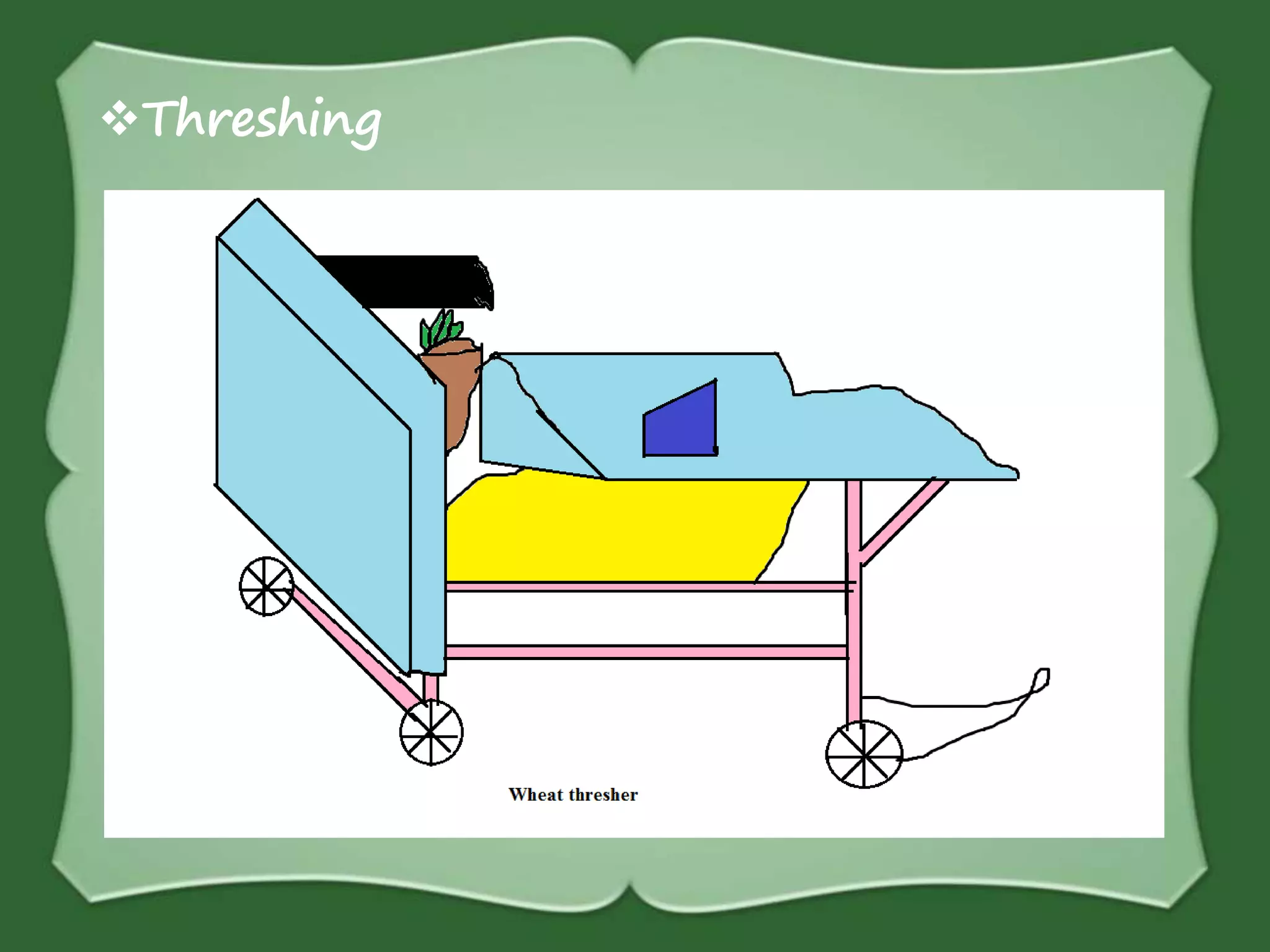
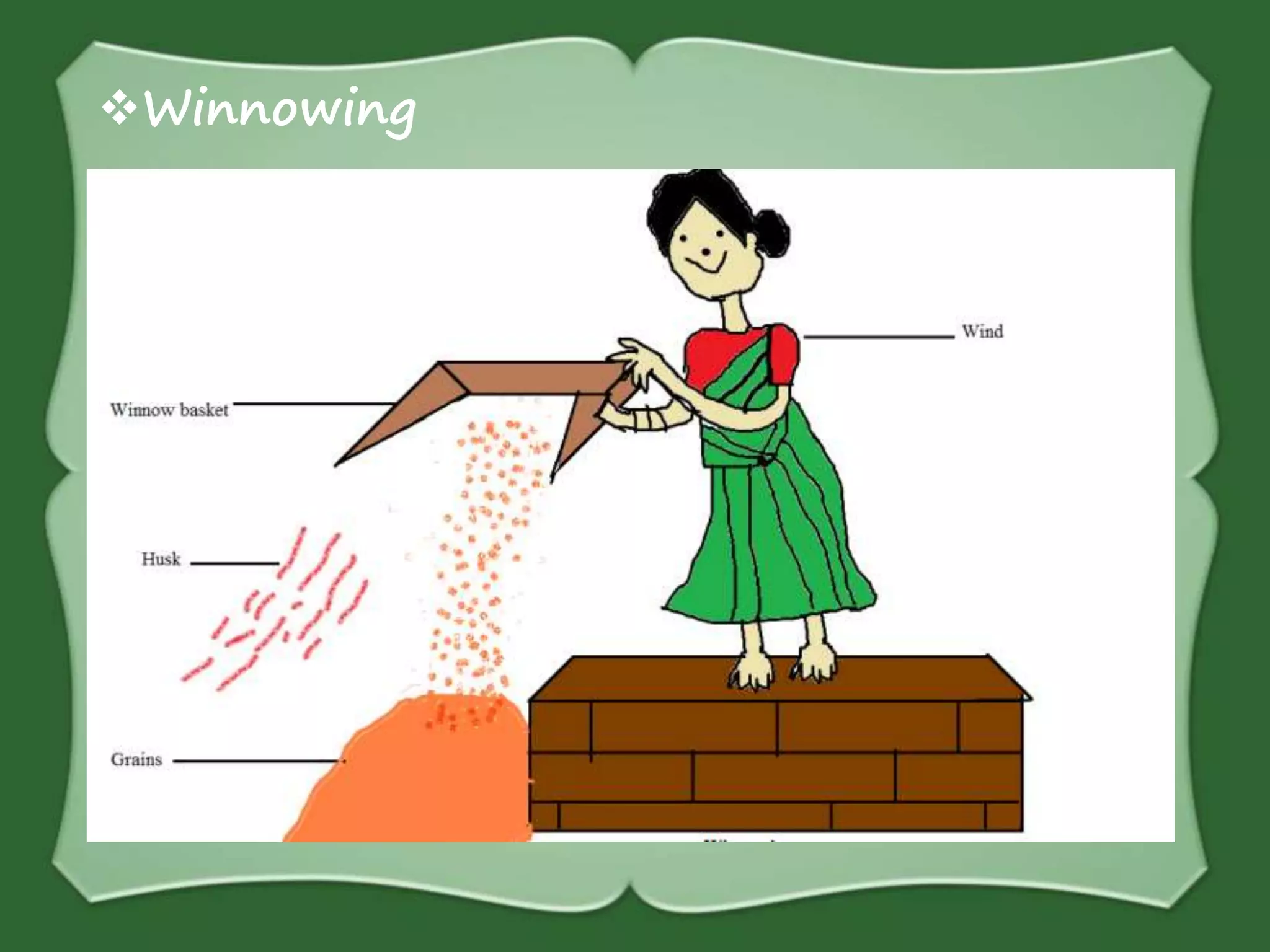
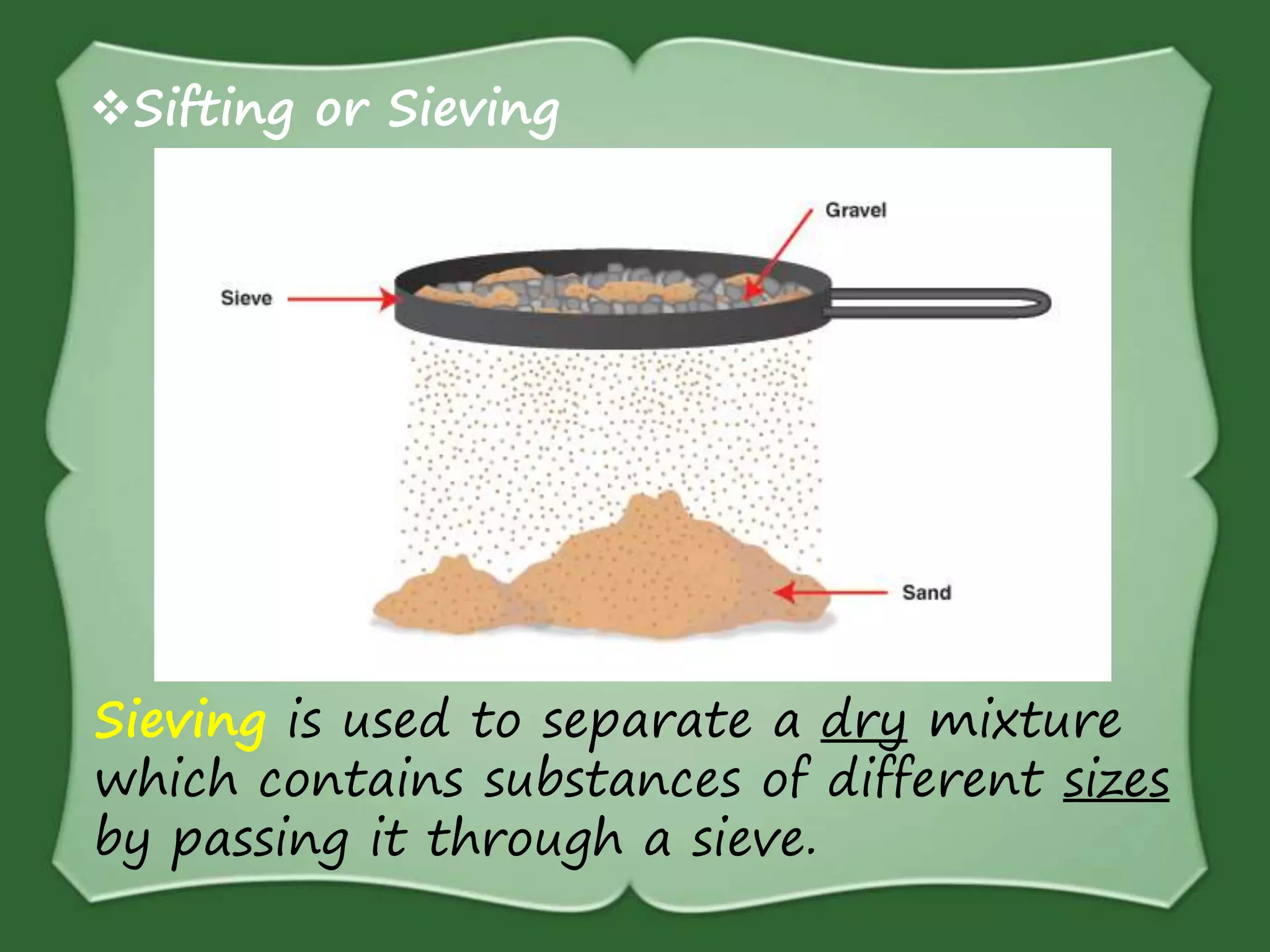
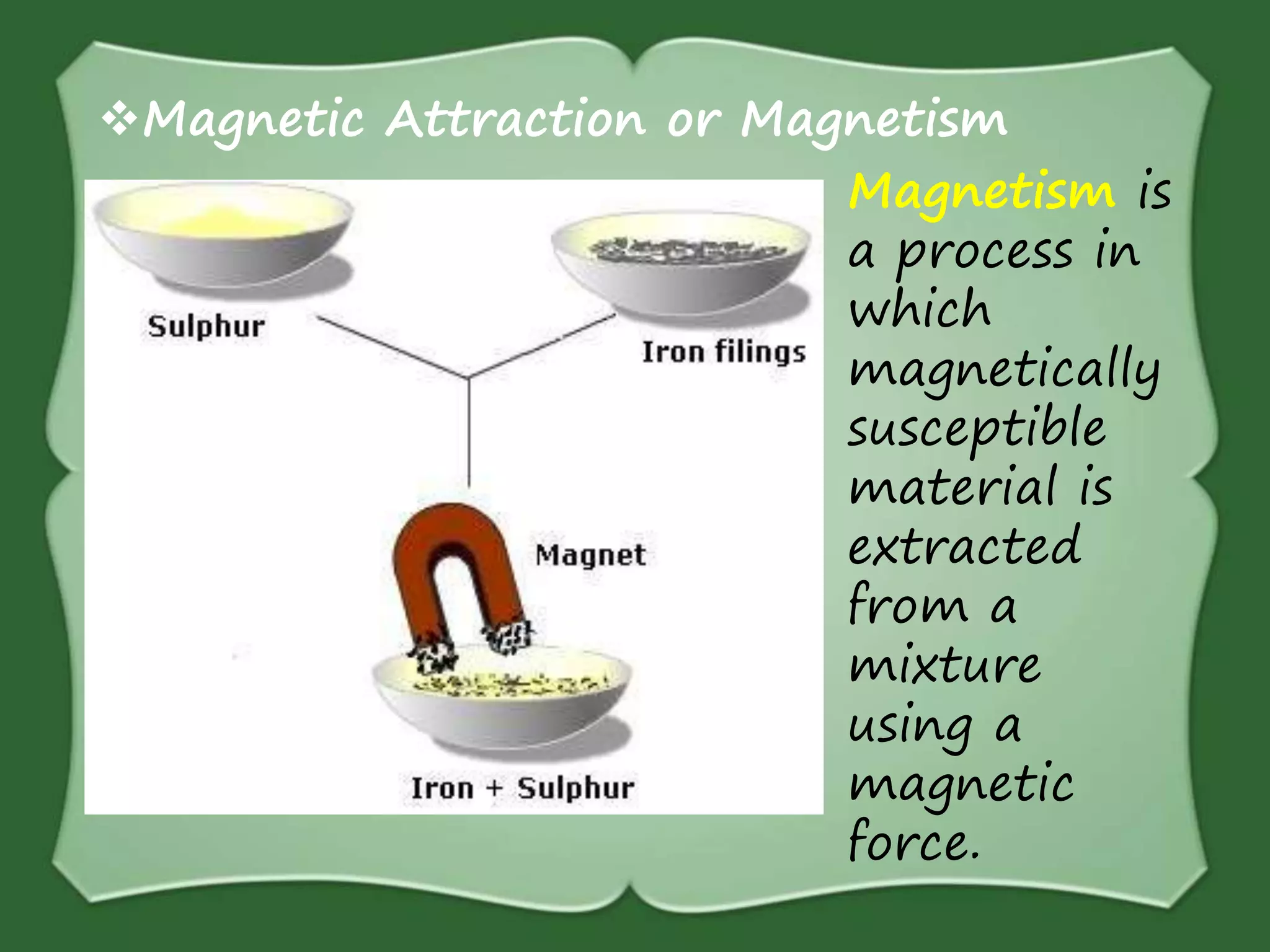
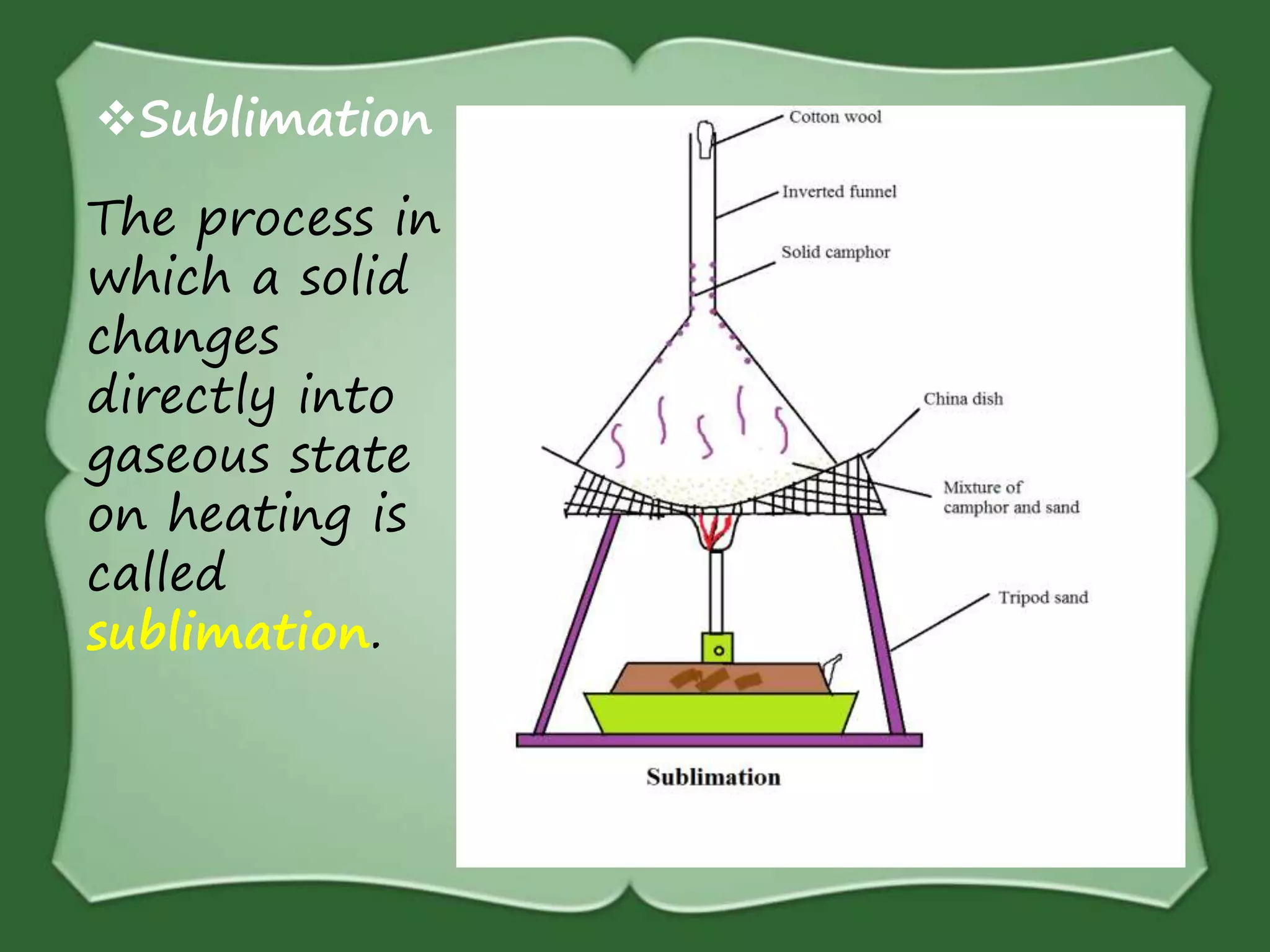
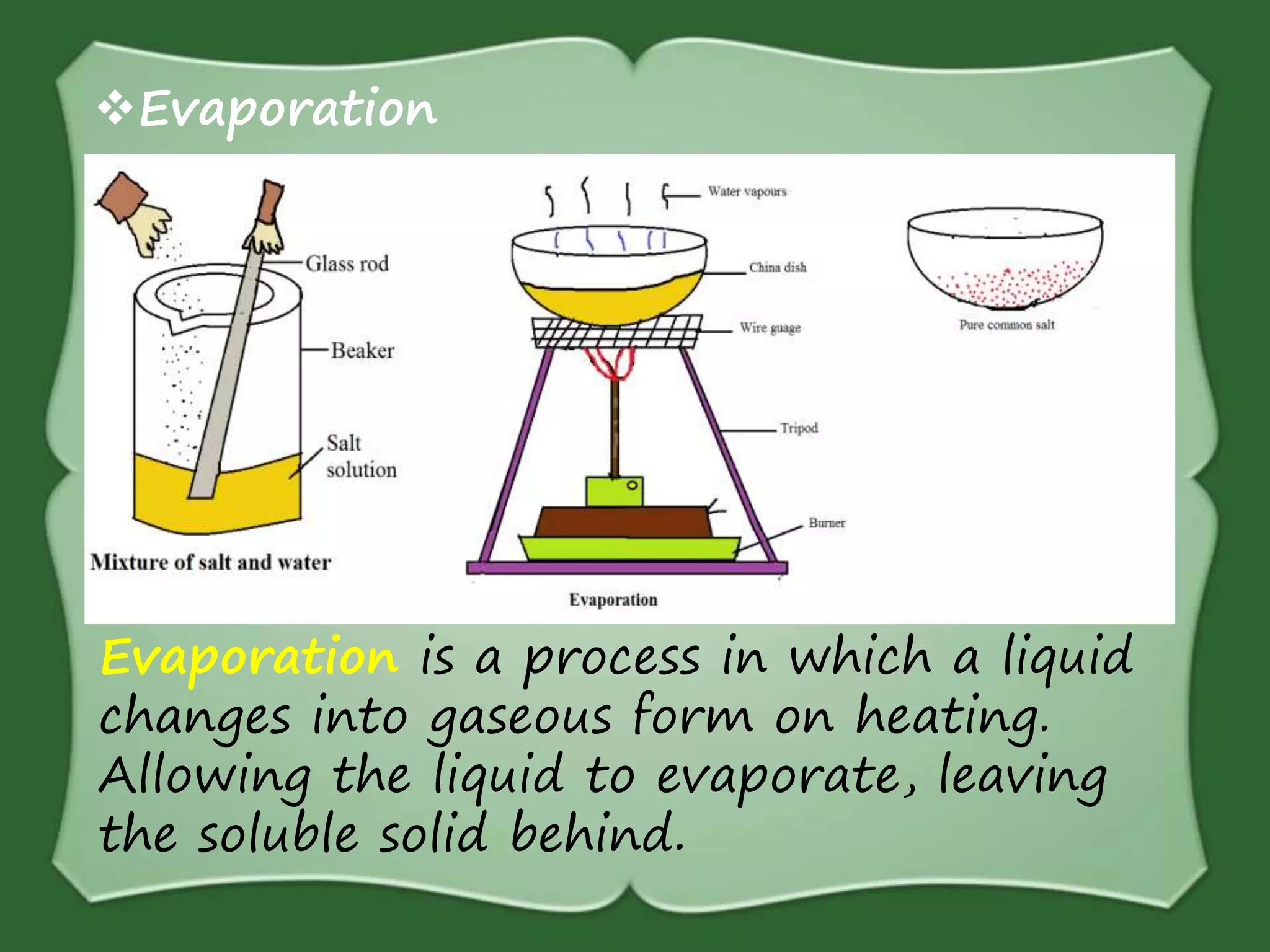
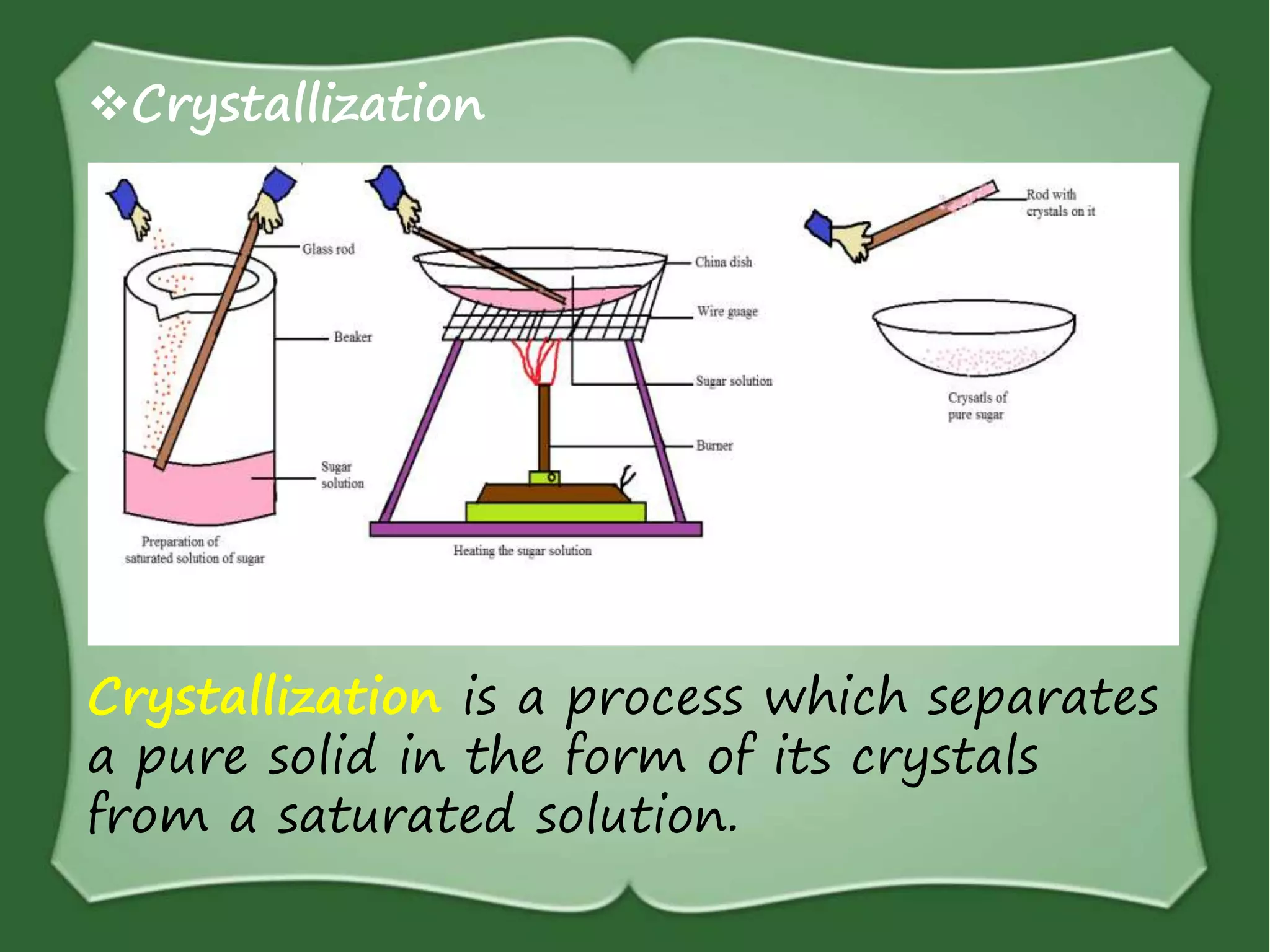
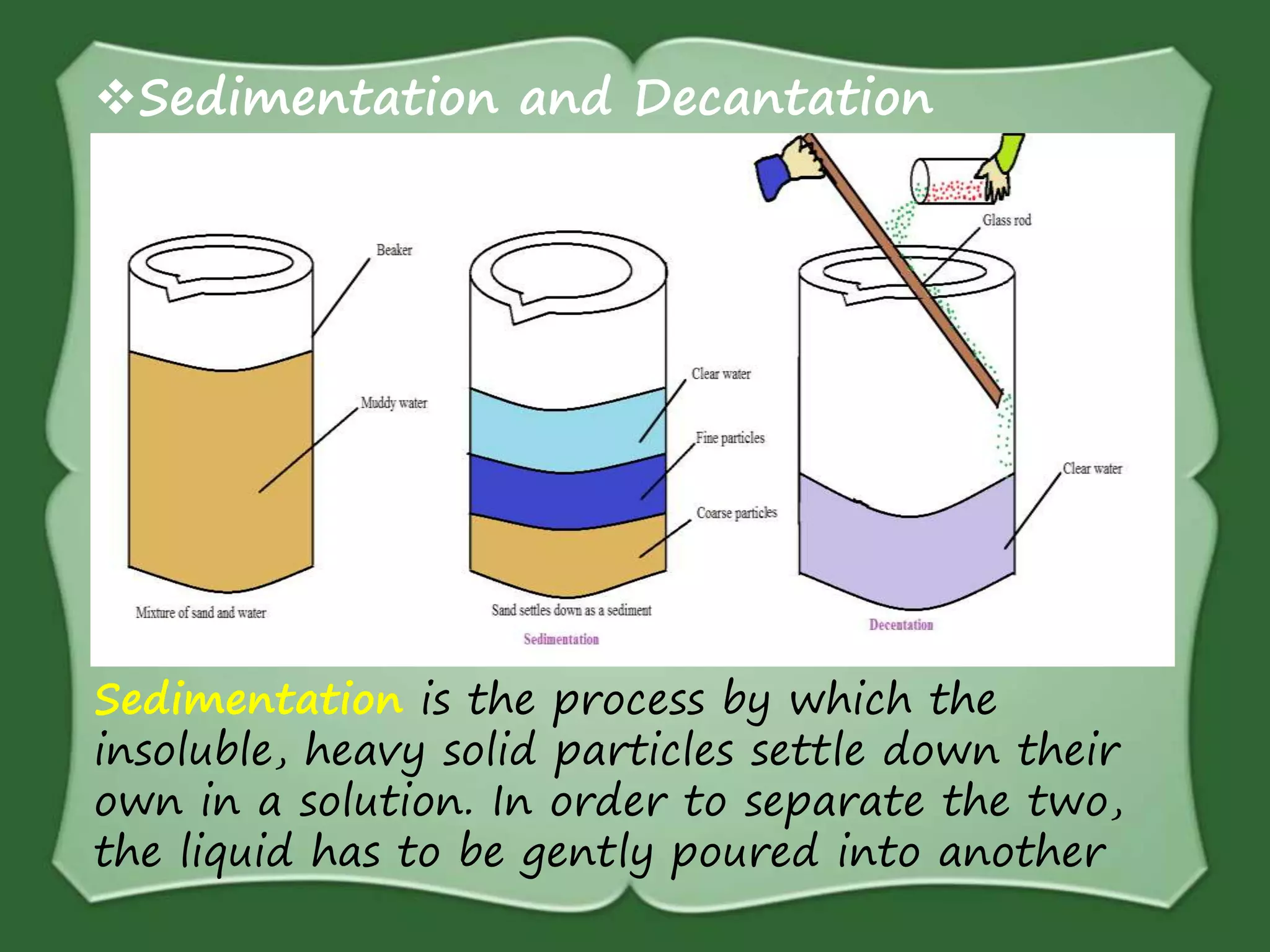
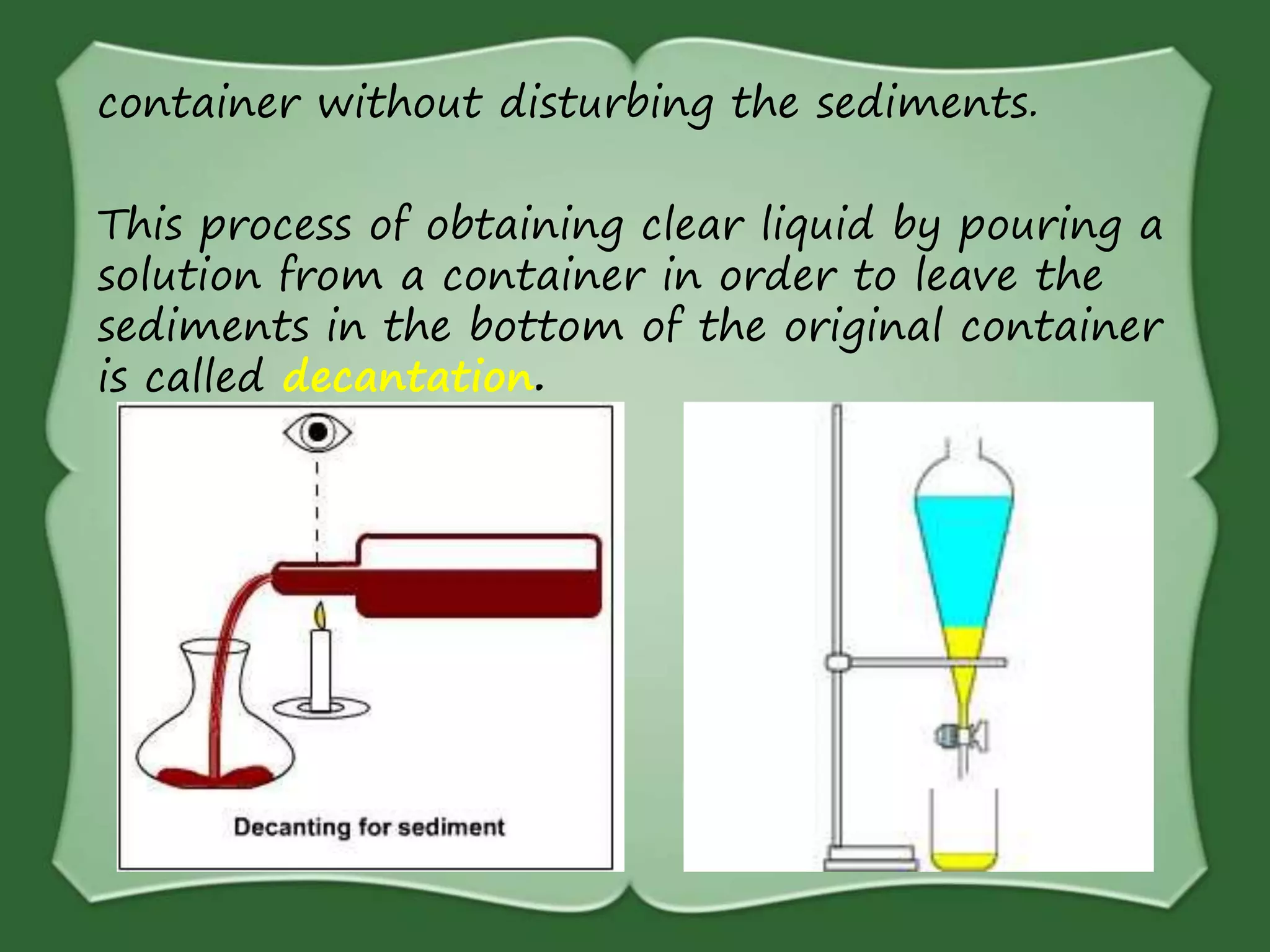
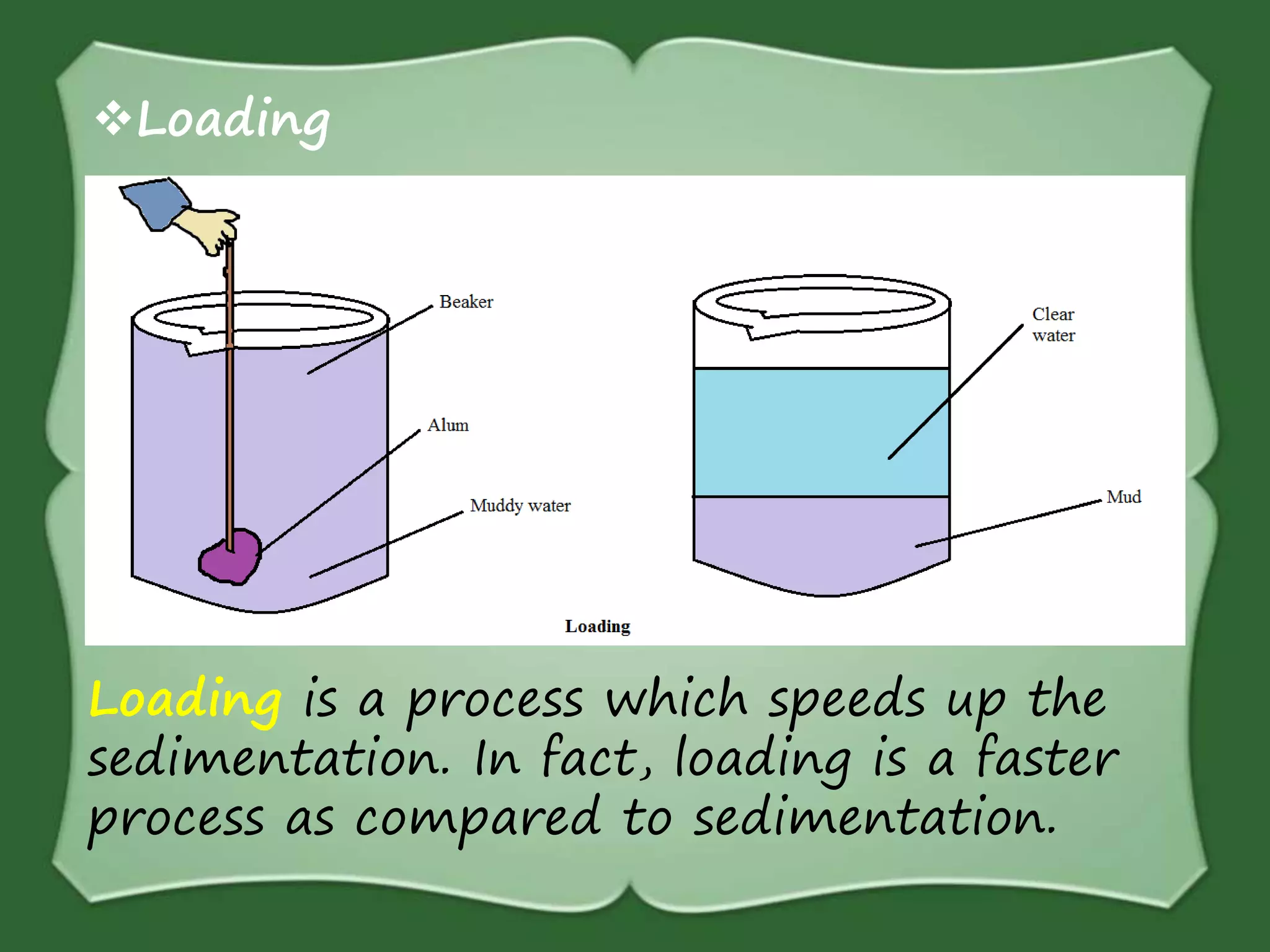
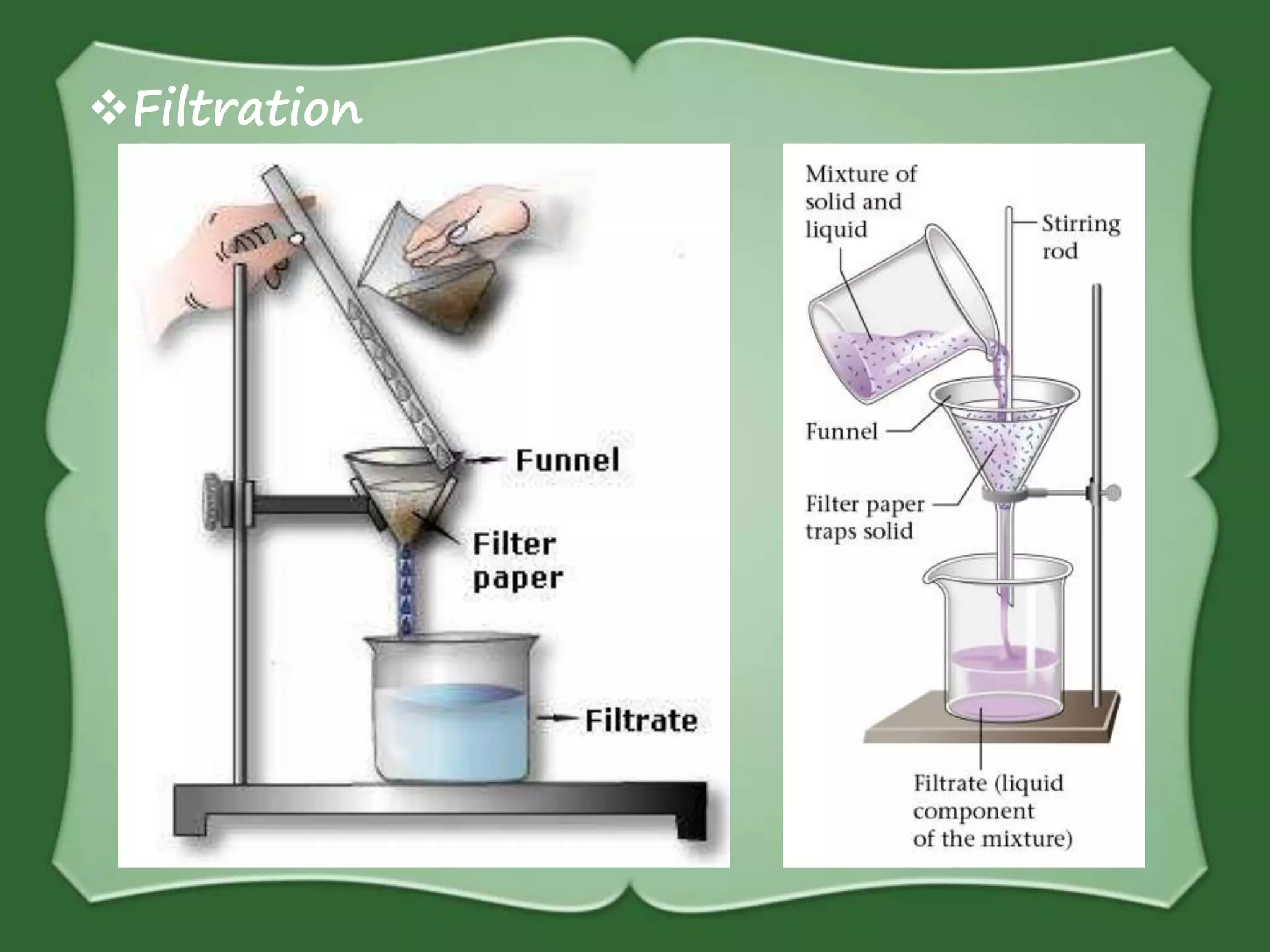
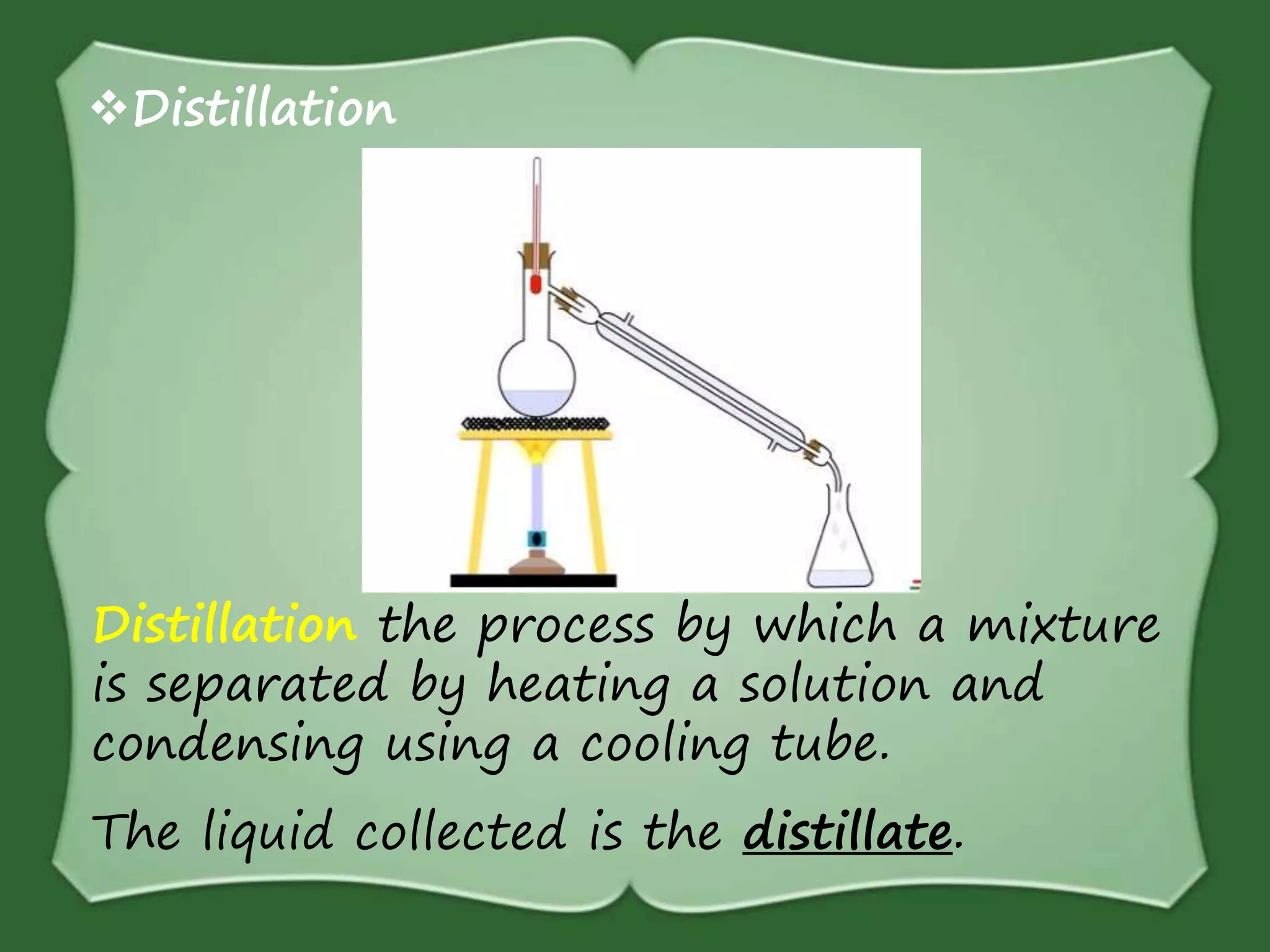
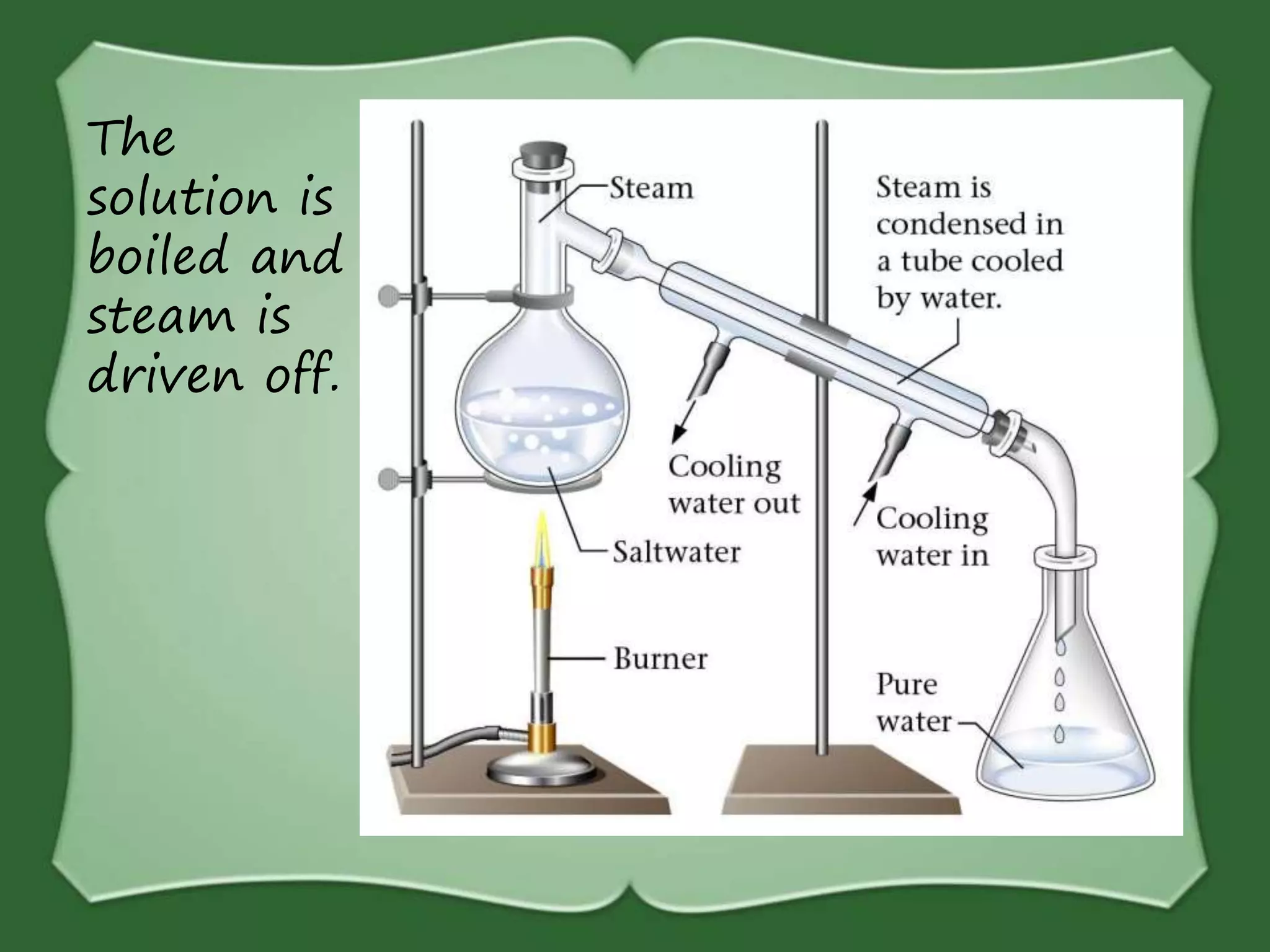
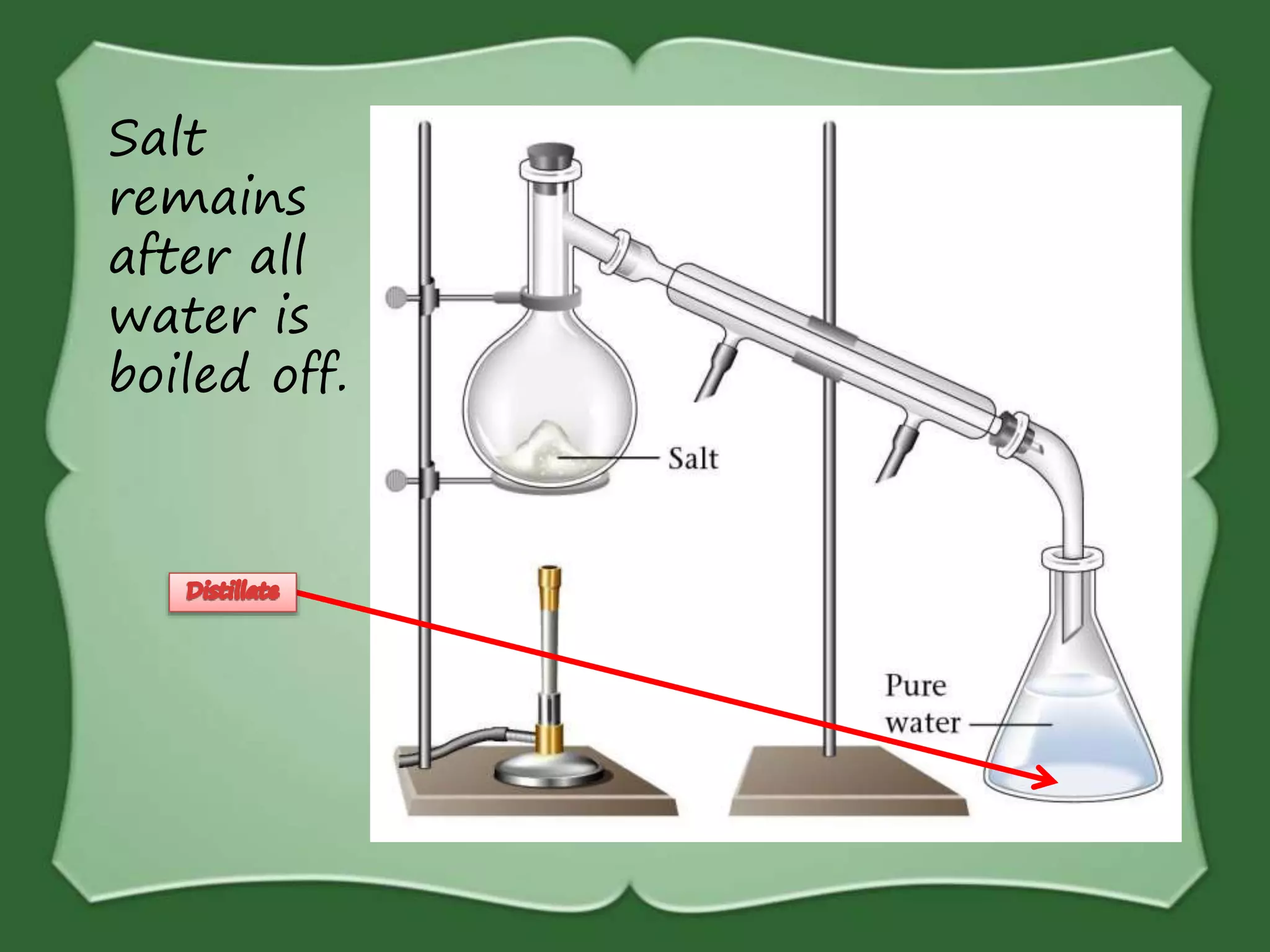
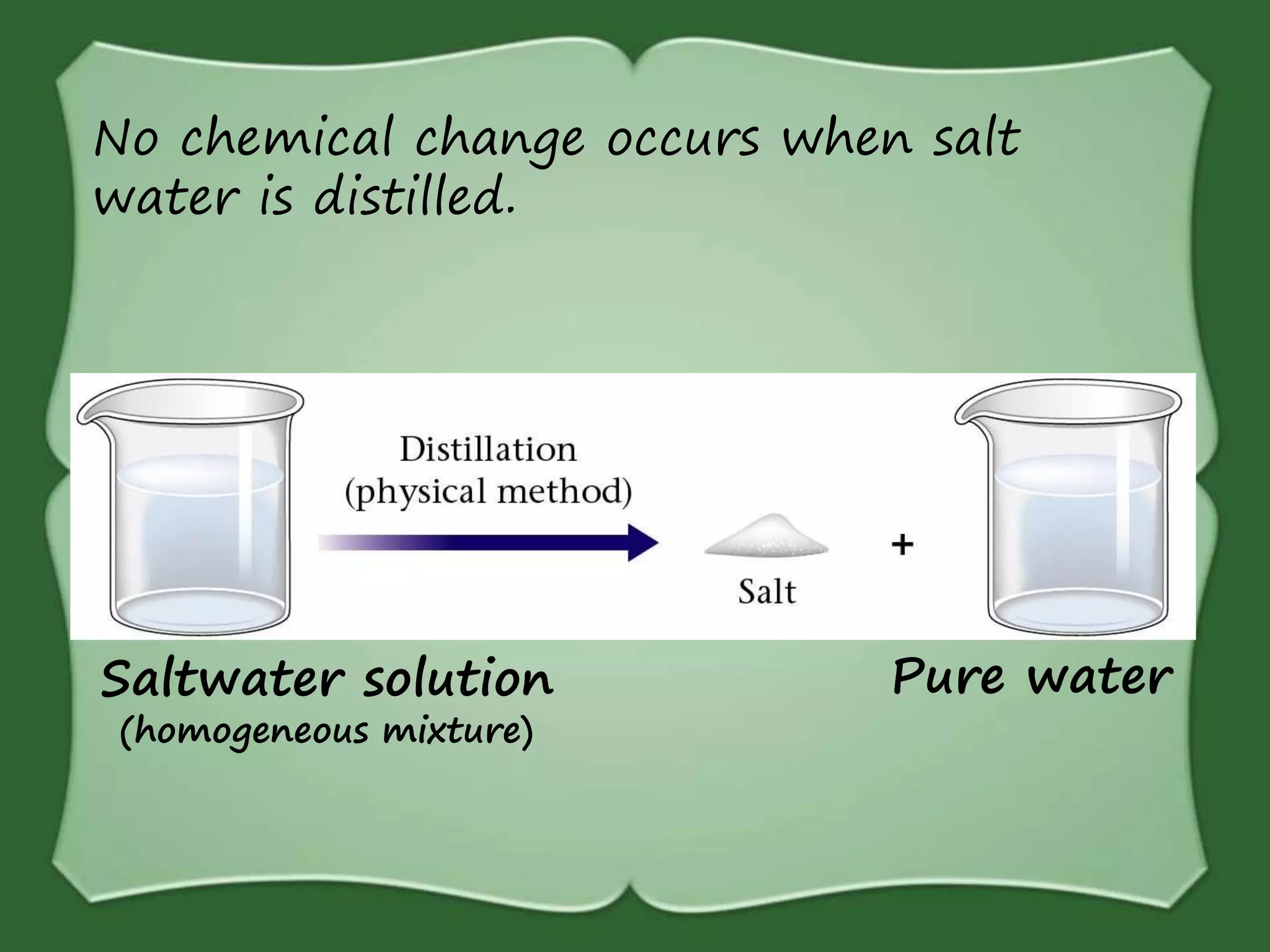
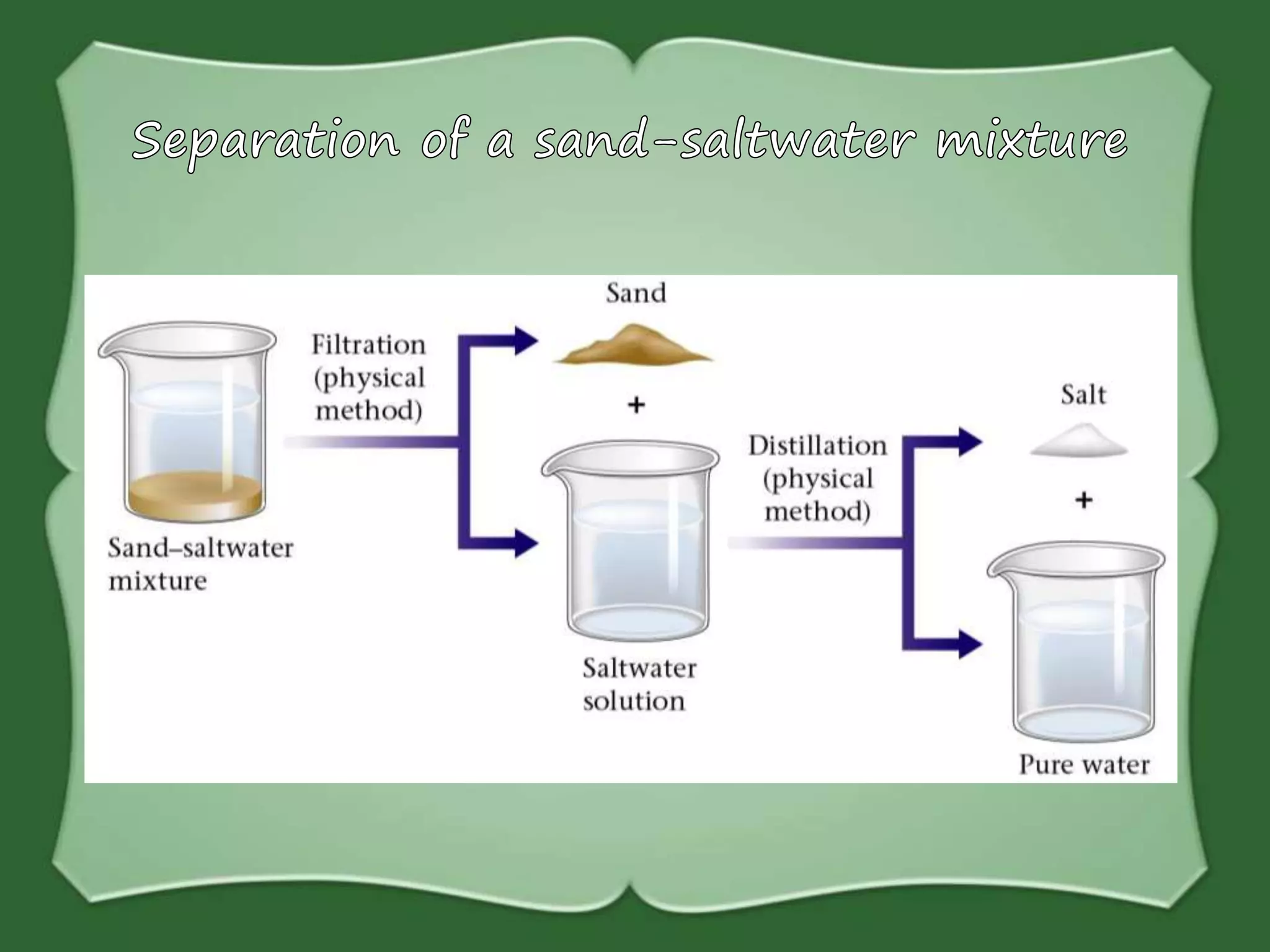
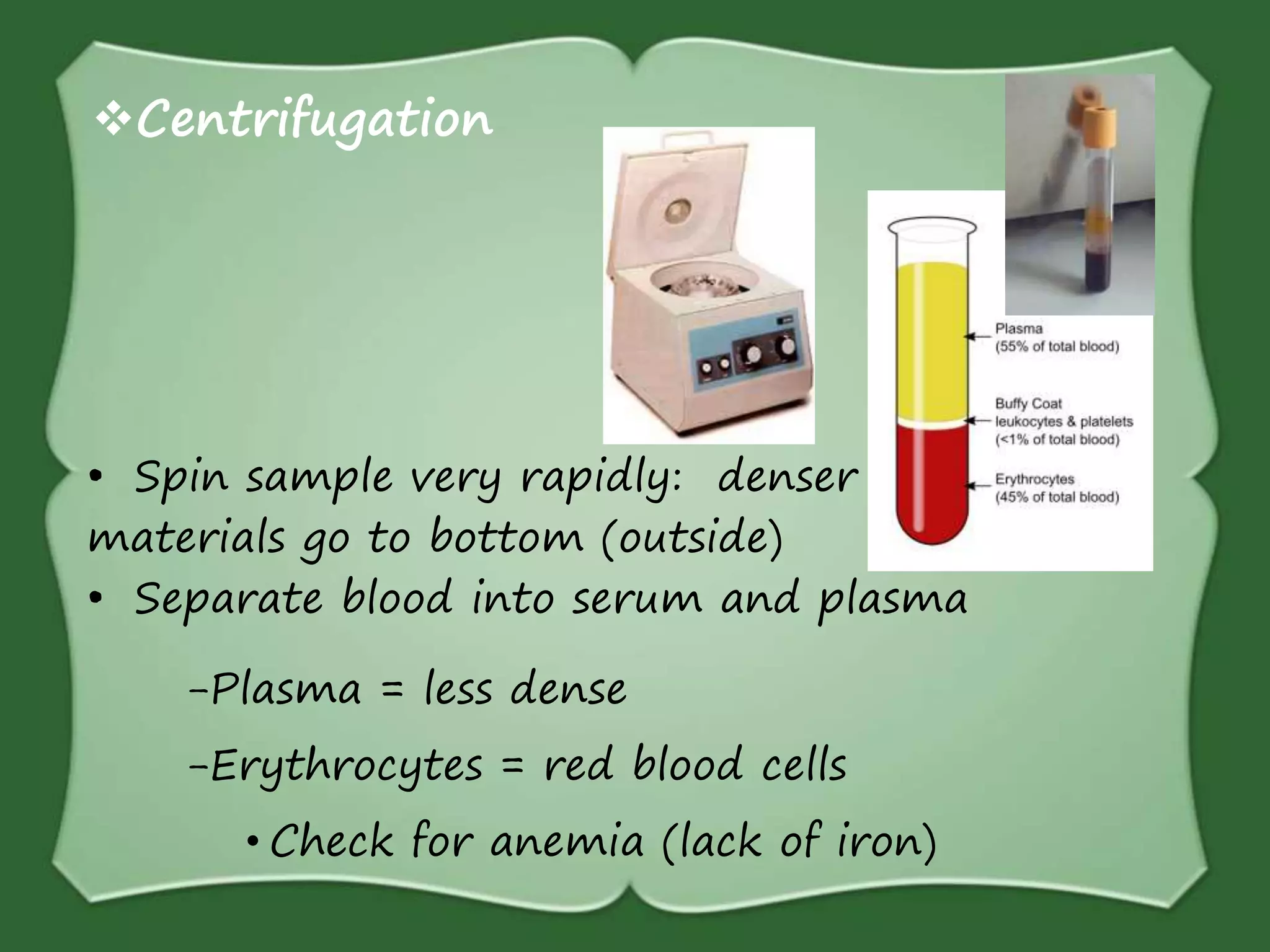
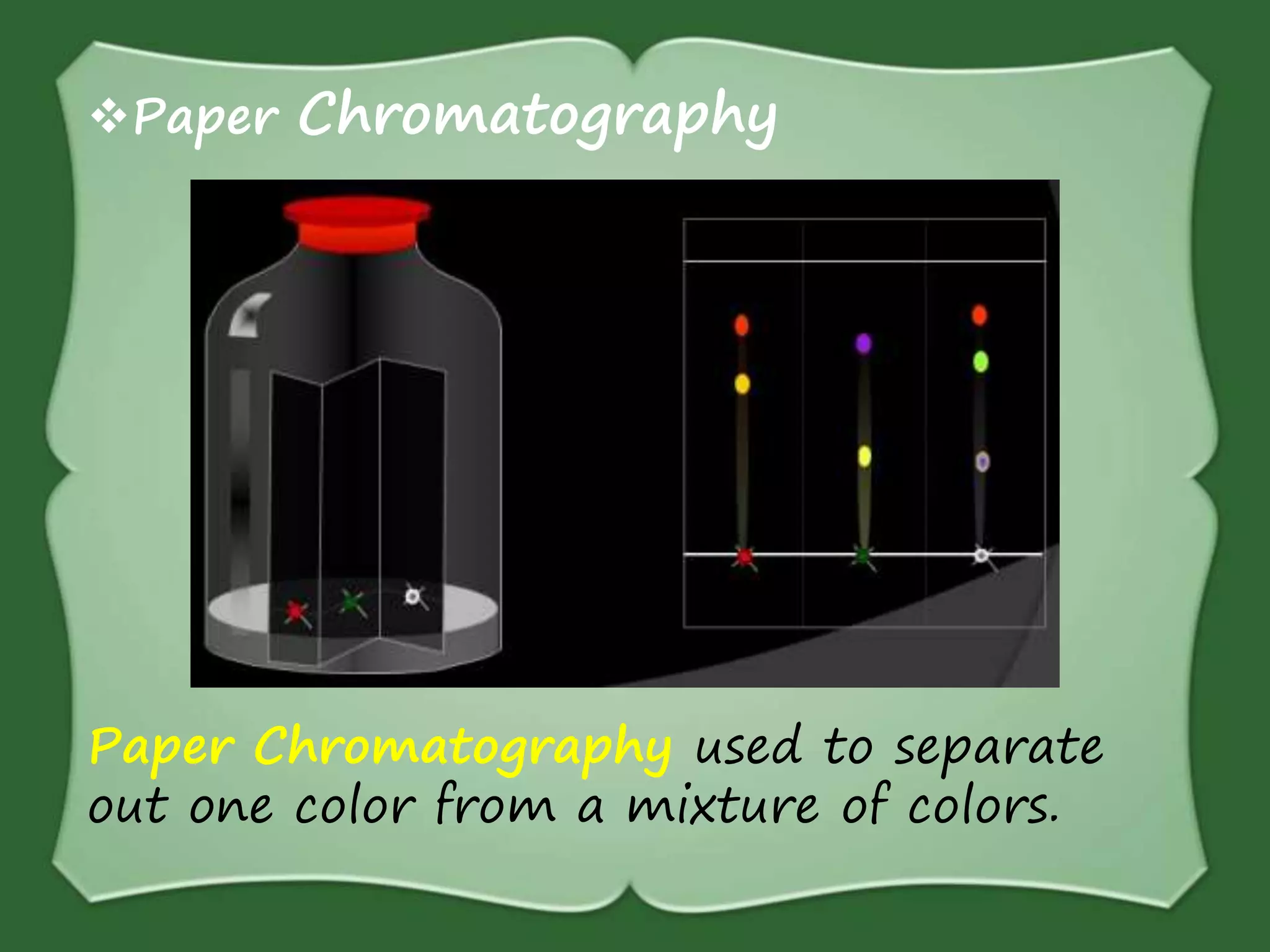
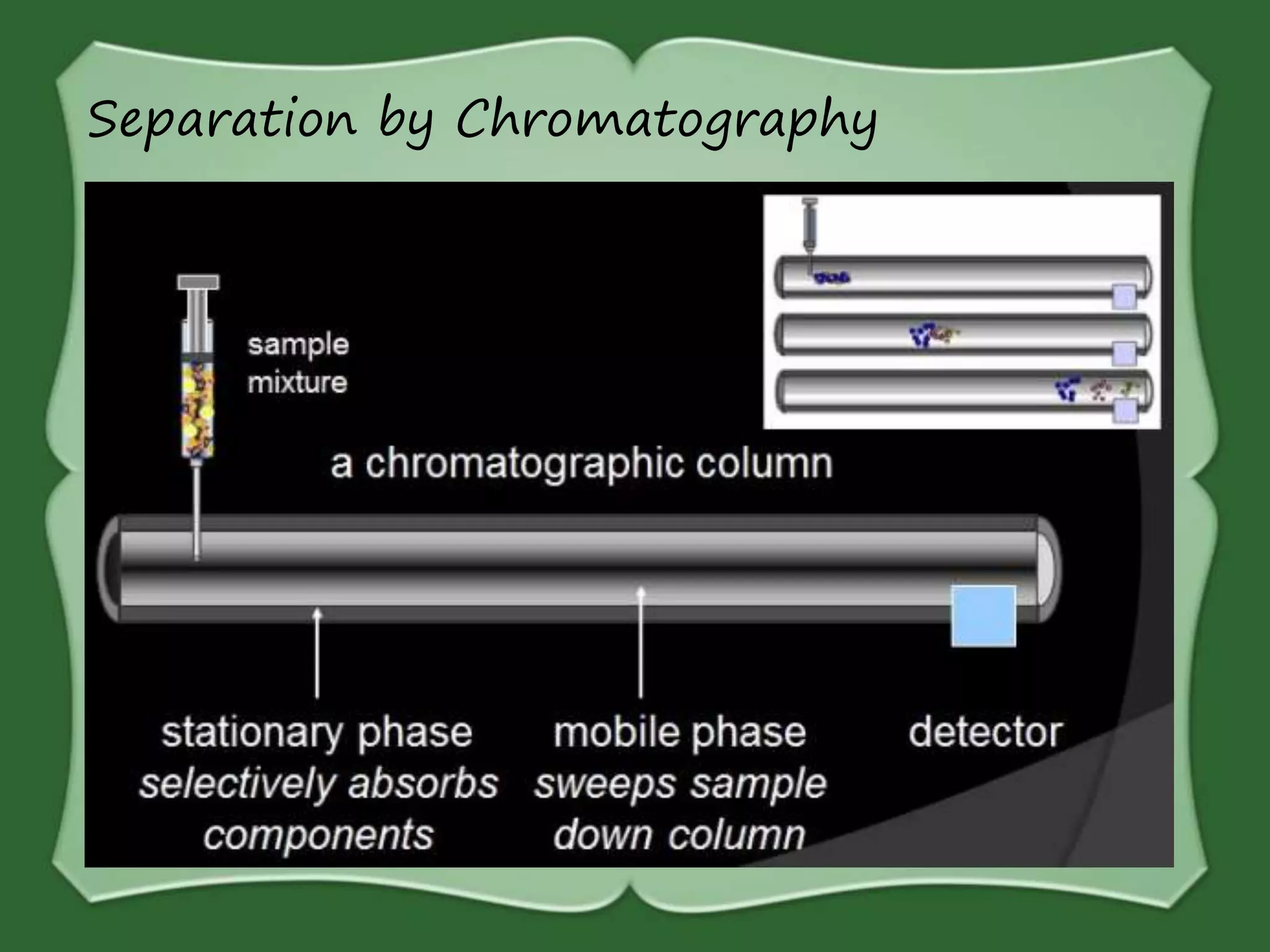
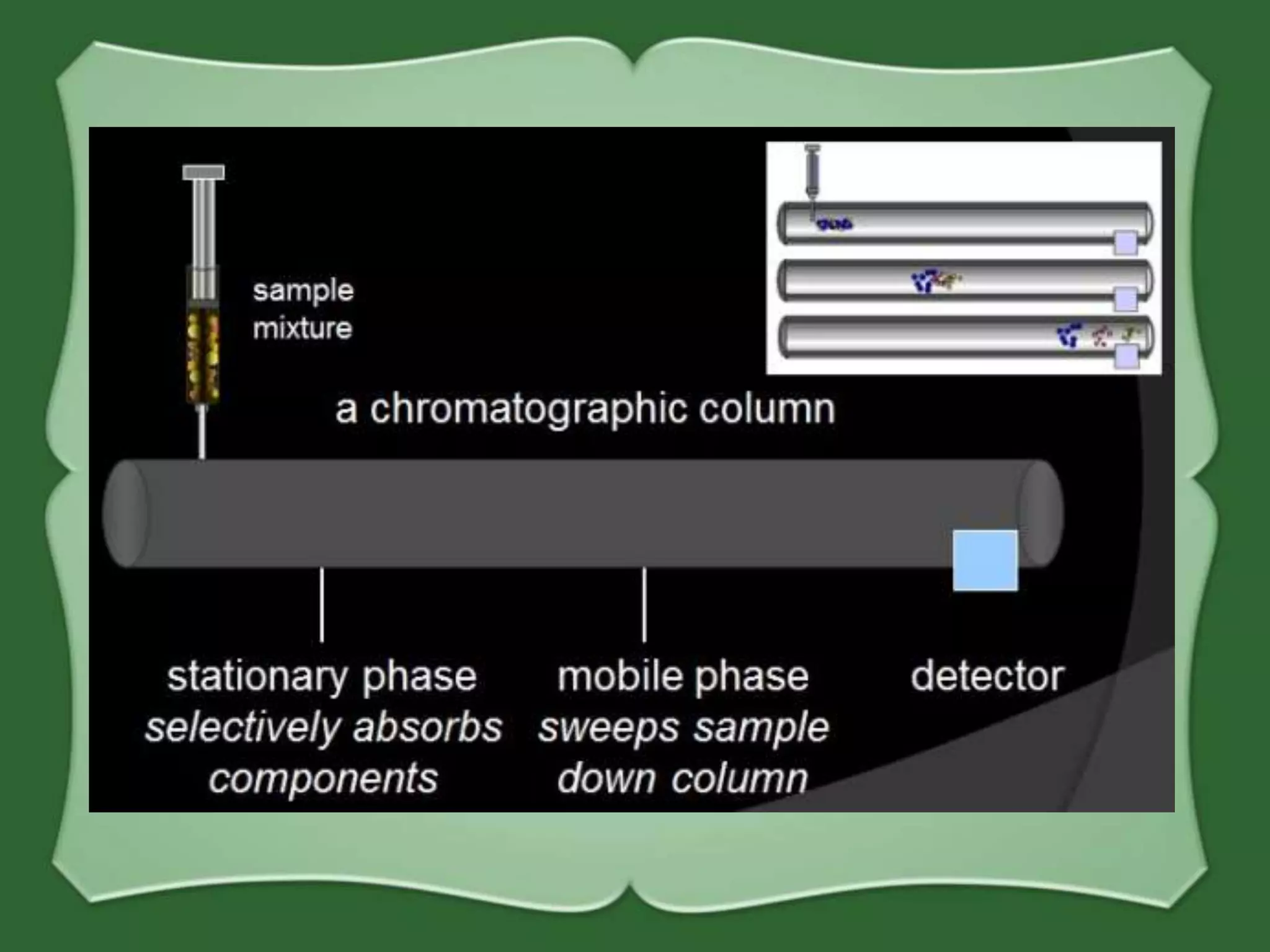
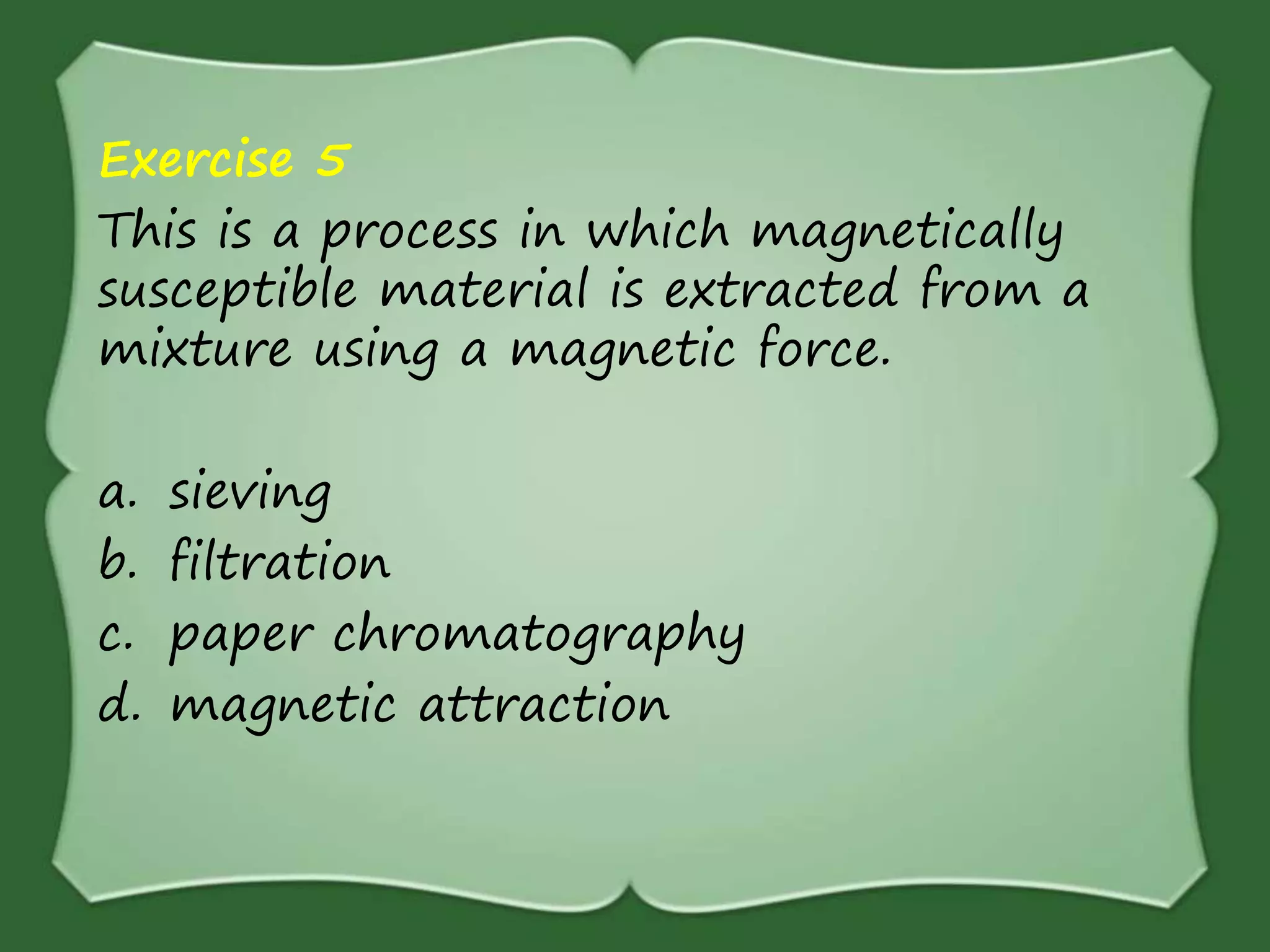
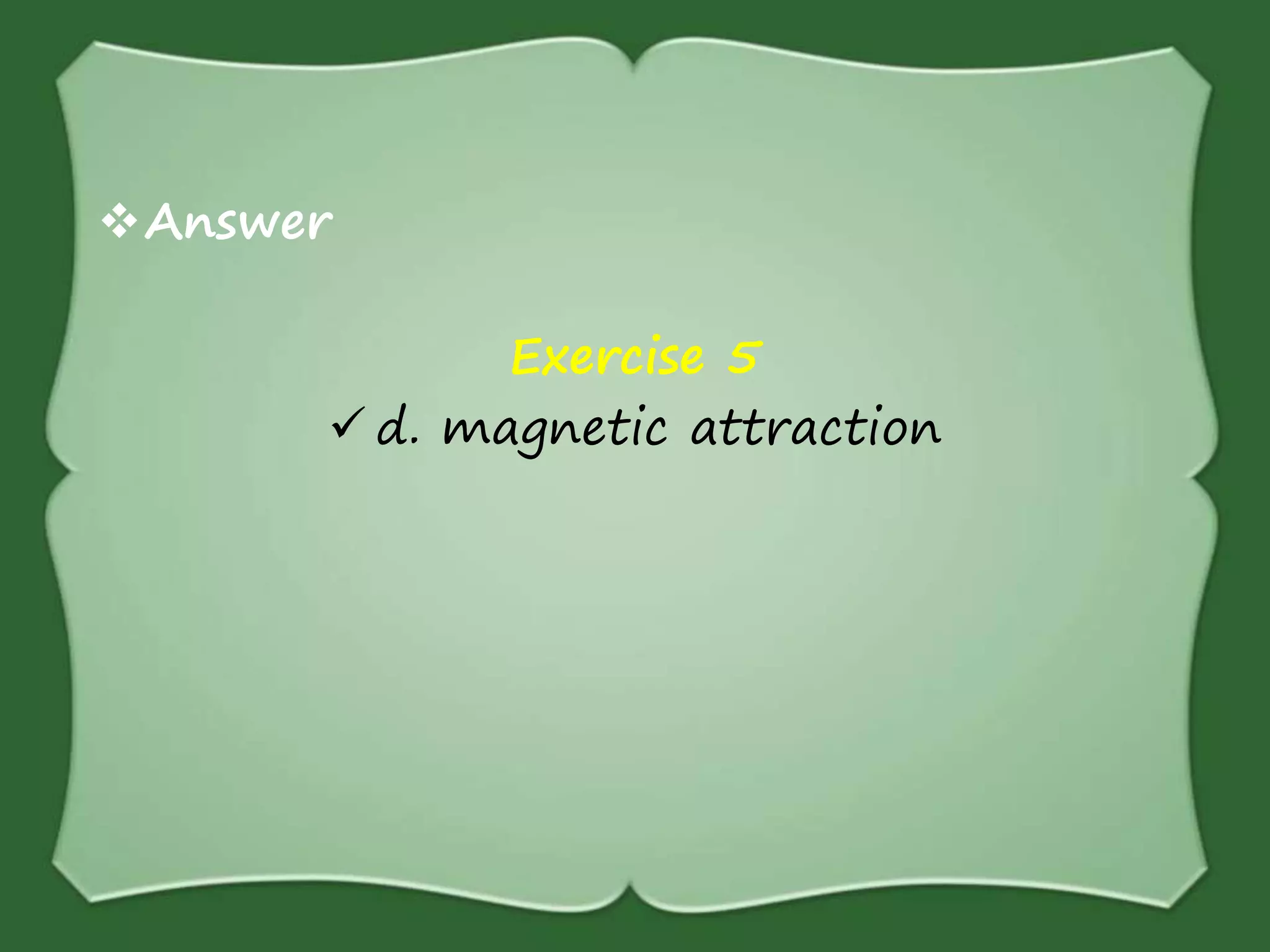
The document discusses various methods for separating mixtures, including hand picking, threshing, winnowing, sieving, magnetic attraction, sublimation, evaporation, crystallization, sedimentation and decantation, loading, filtration, distillation, centrifugation, and paper chromatography. It defines heterogeneous mixtures as mixtures whose components are not uniformly distributed and can be distinguished from each other, and homogeneous mixtures as mixtures whose components are uniformly distributed and cannot be distinguished from each other. Examples are given of how these separation methods can be used in daily life and industry.