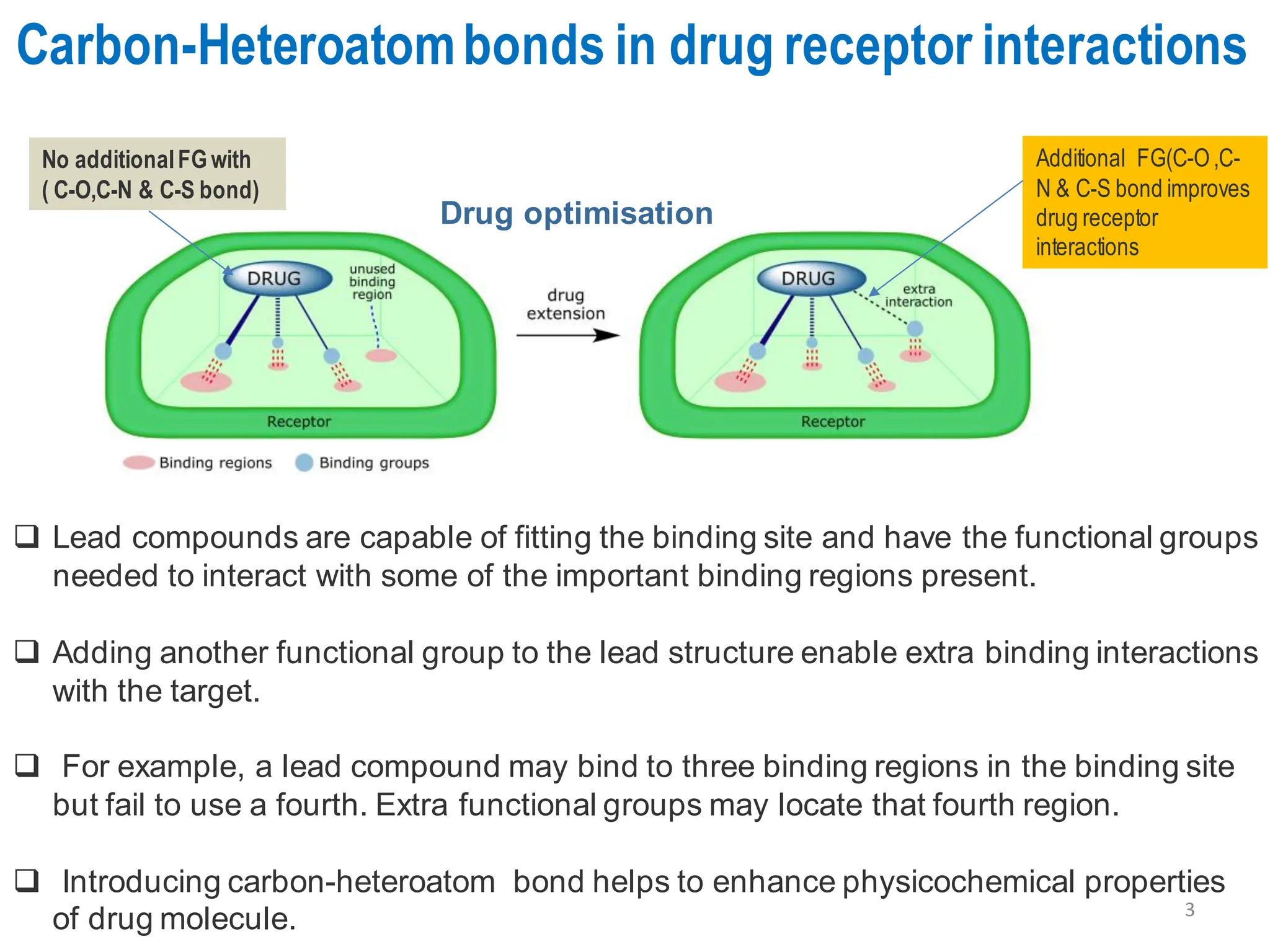
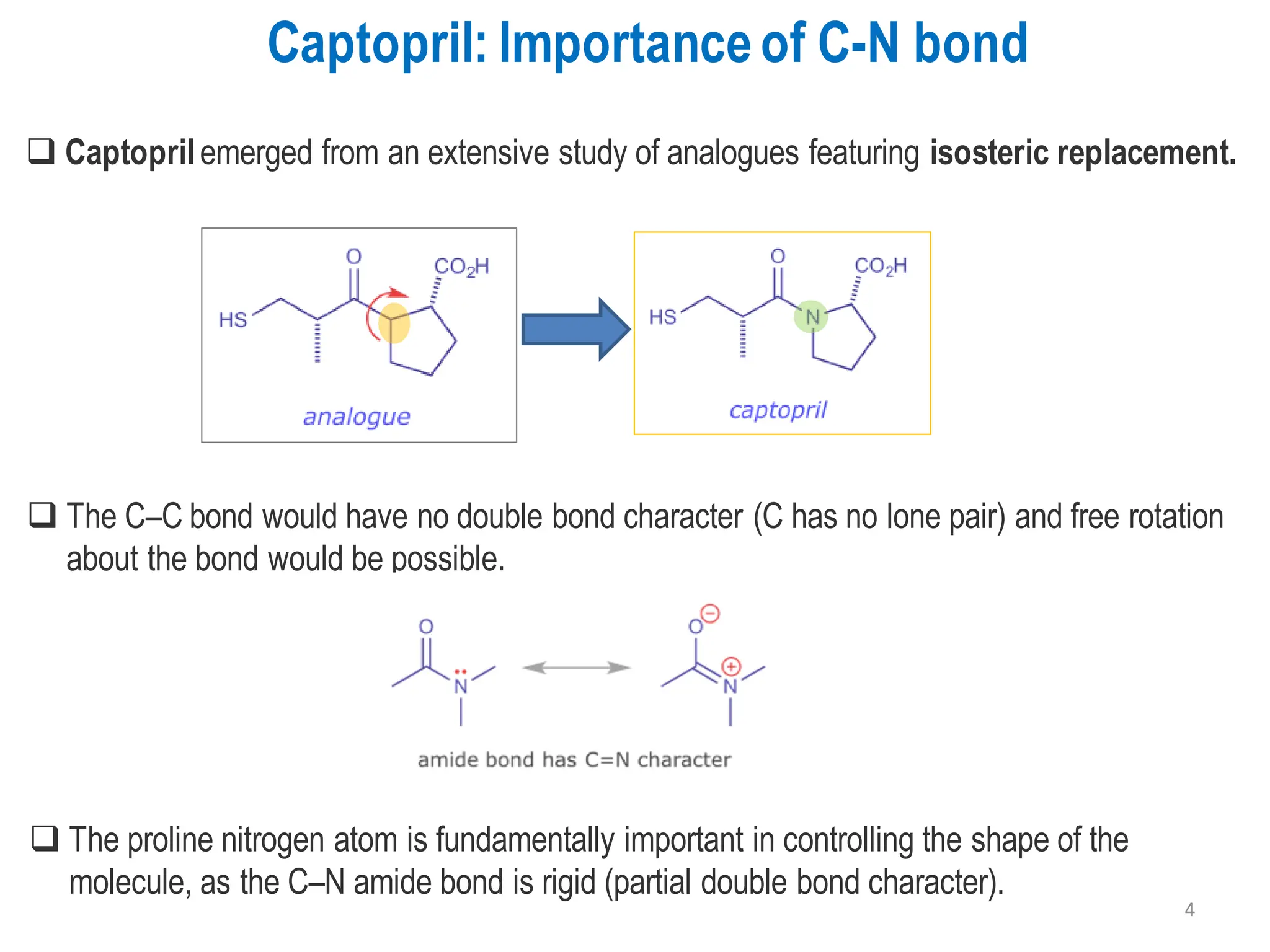
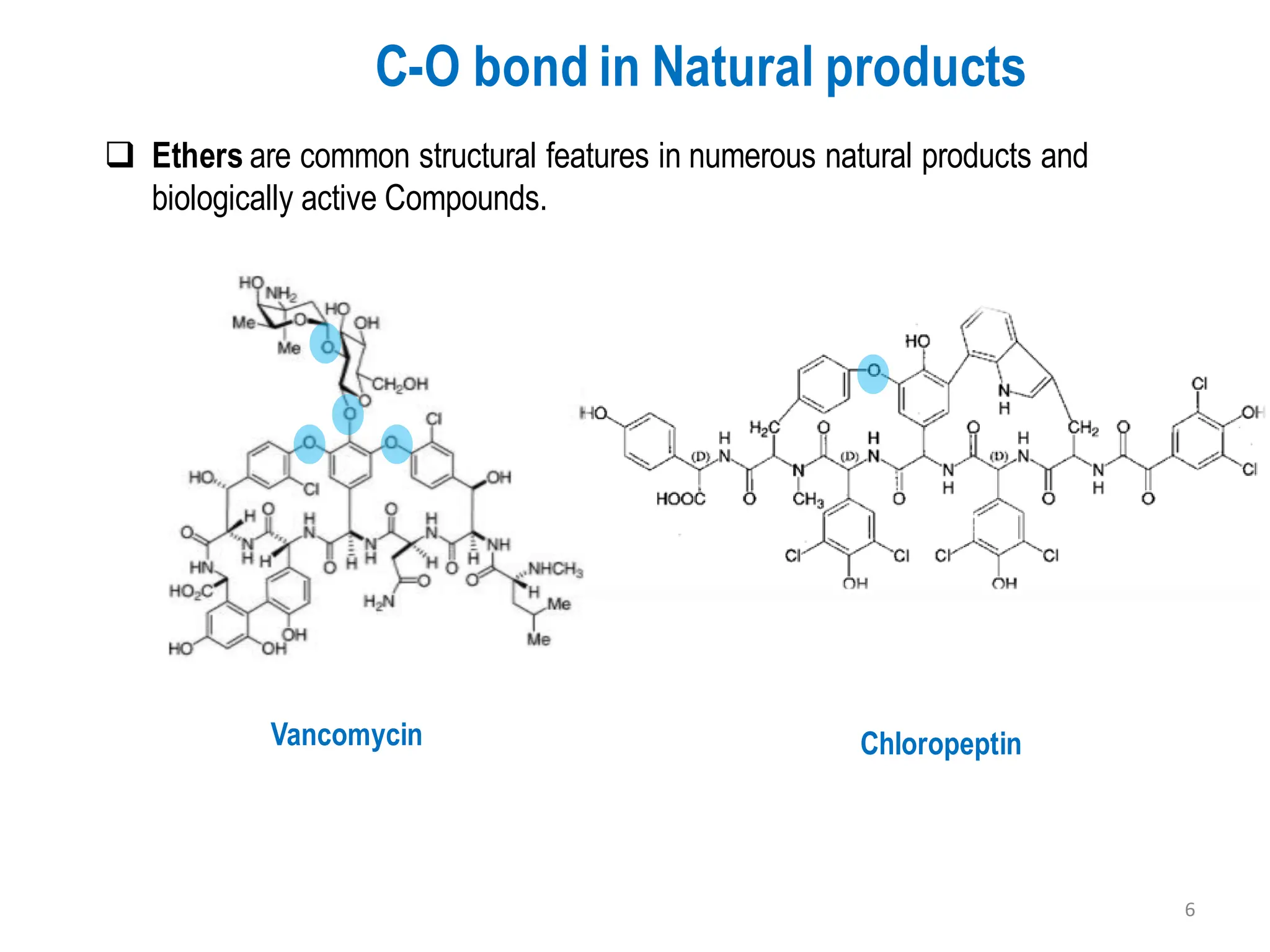
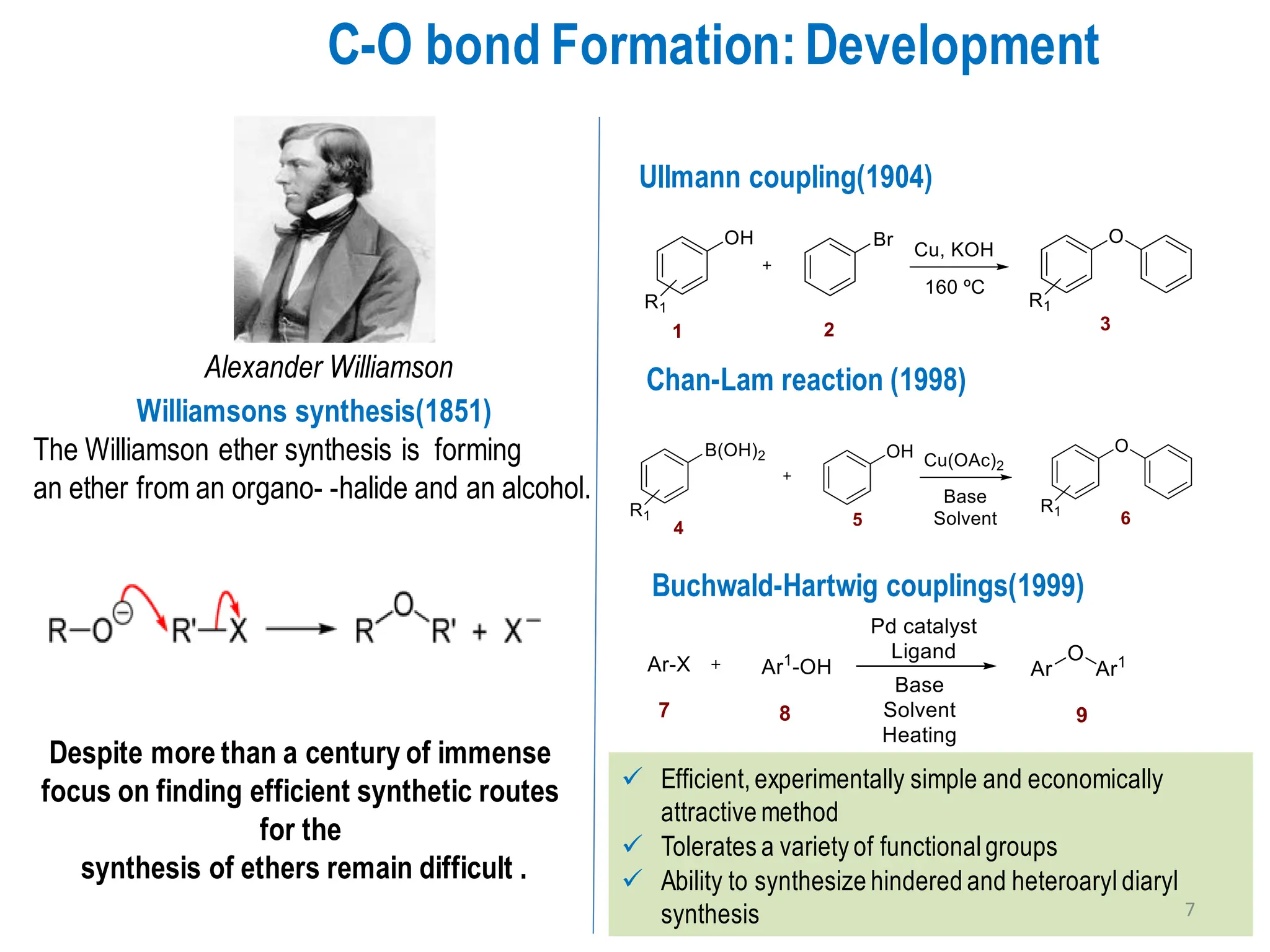
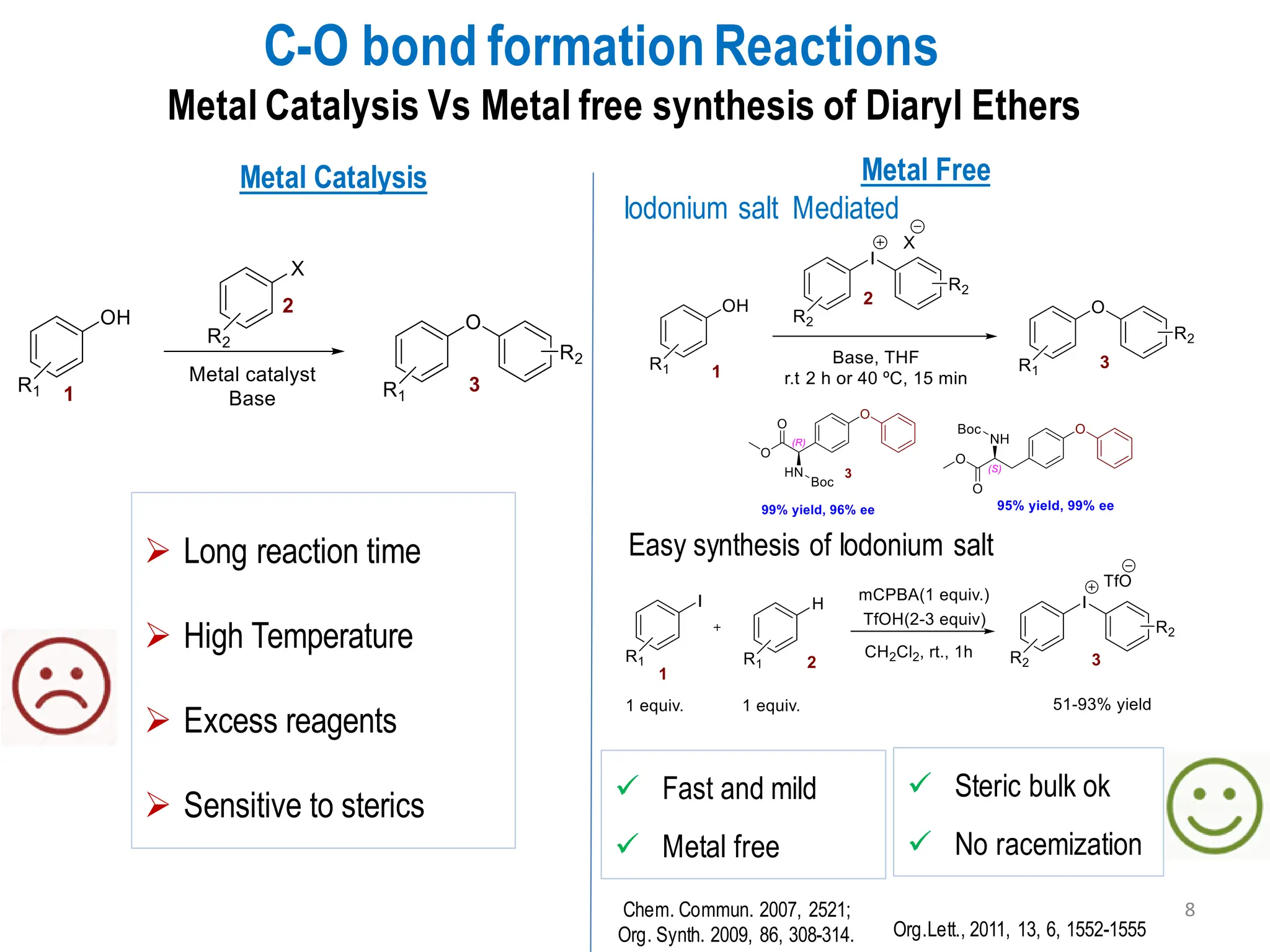
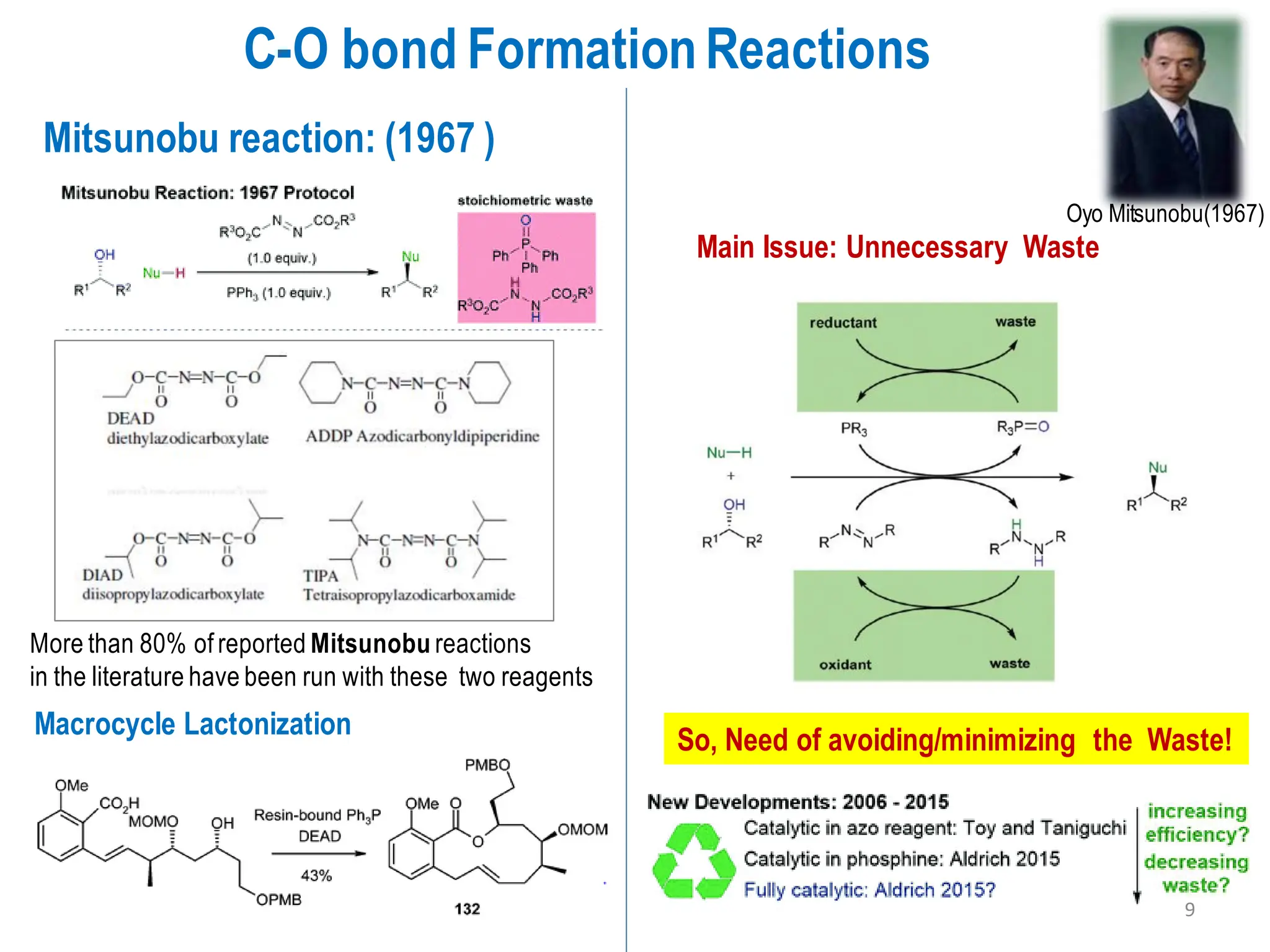
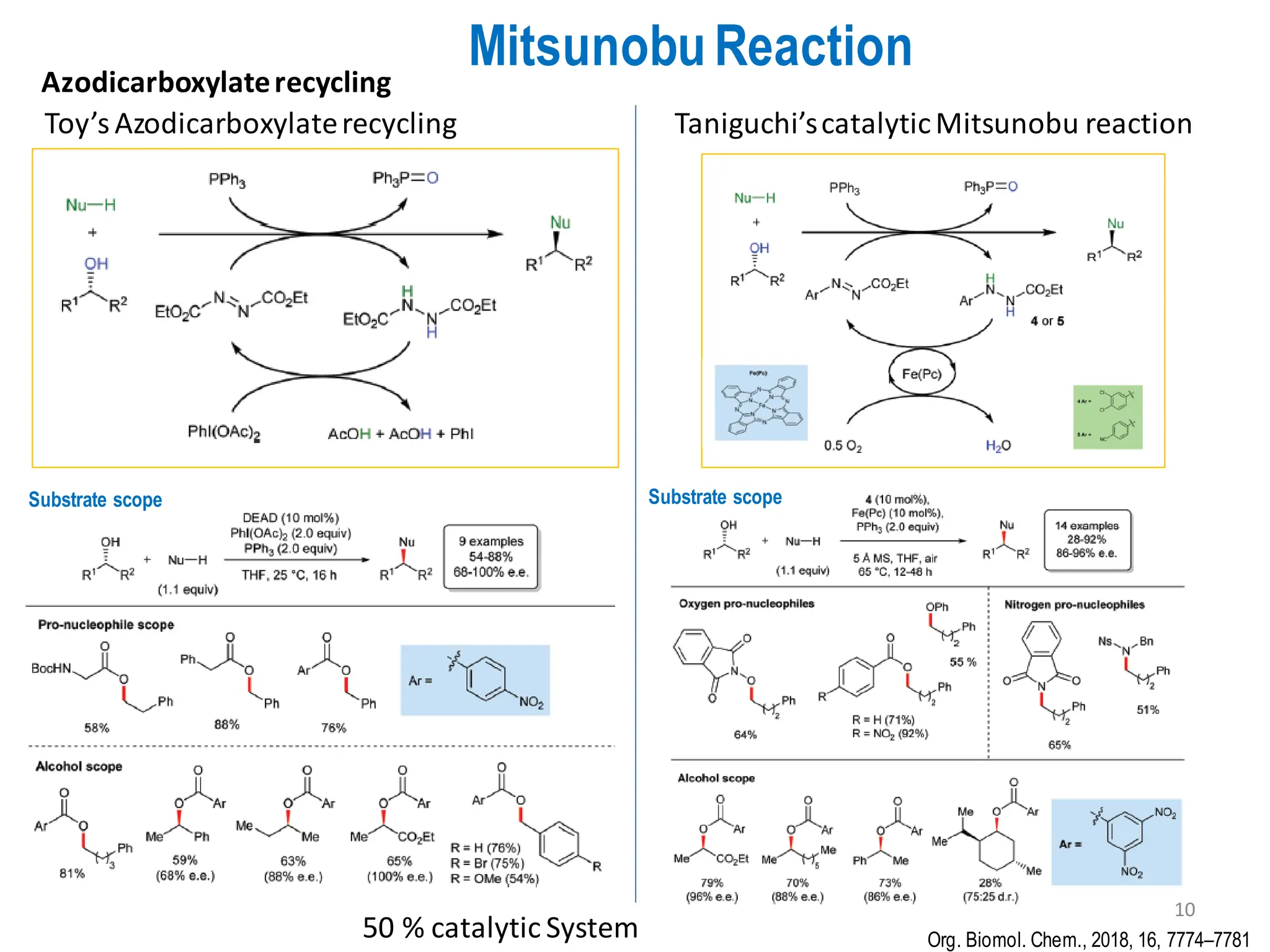
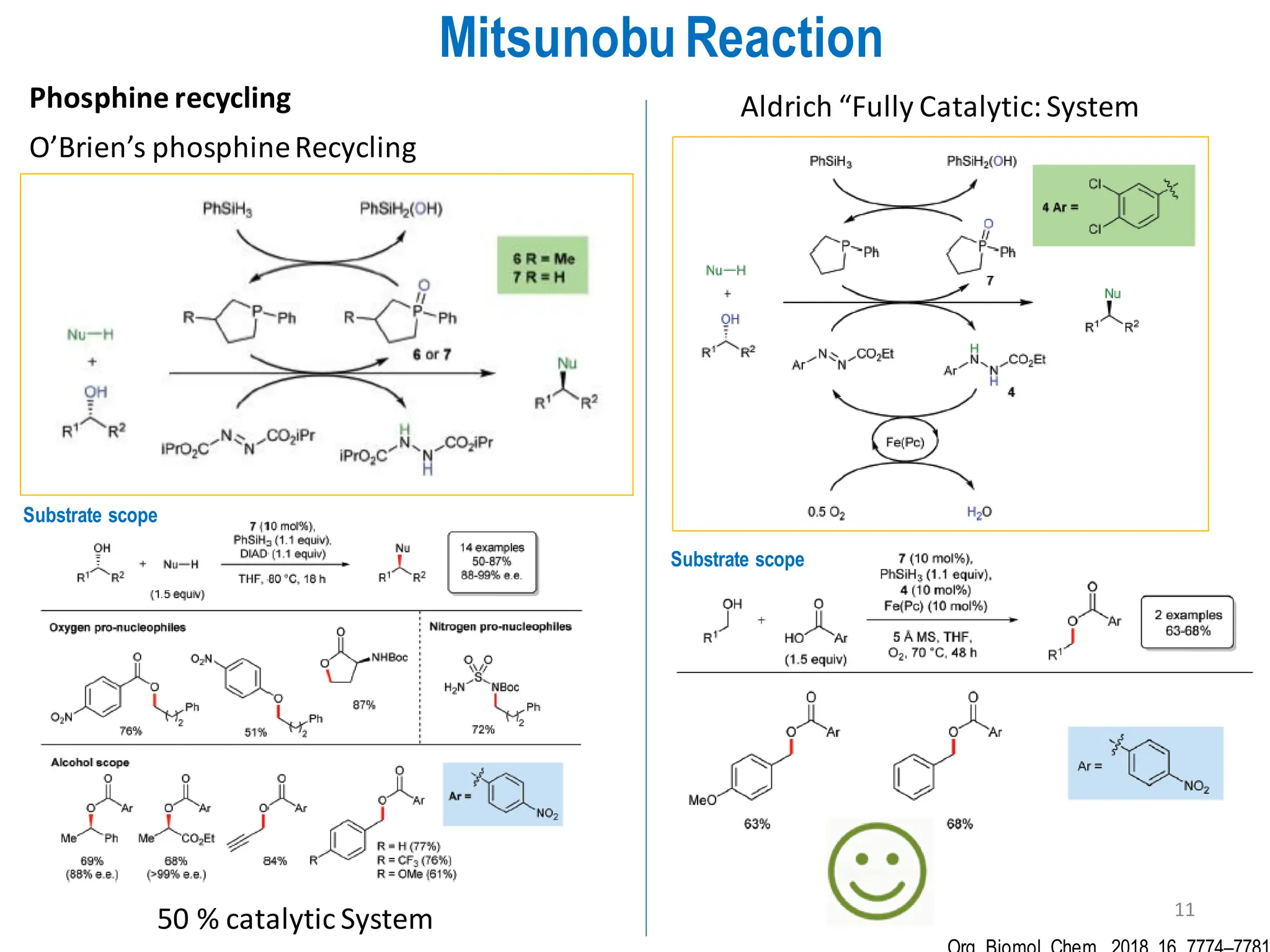
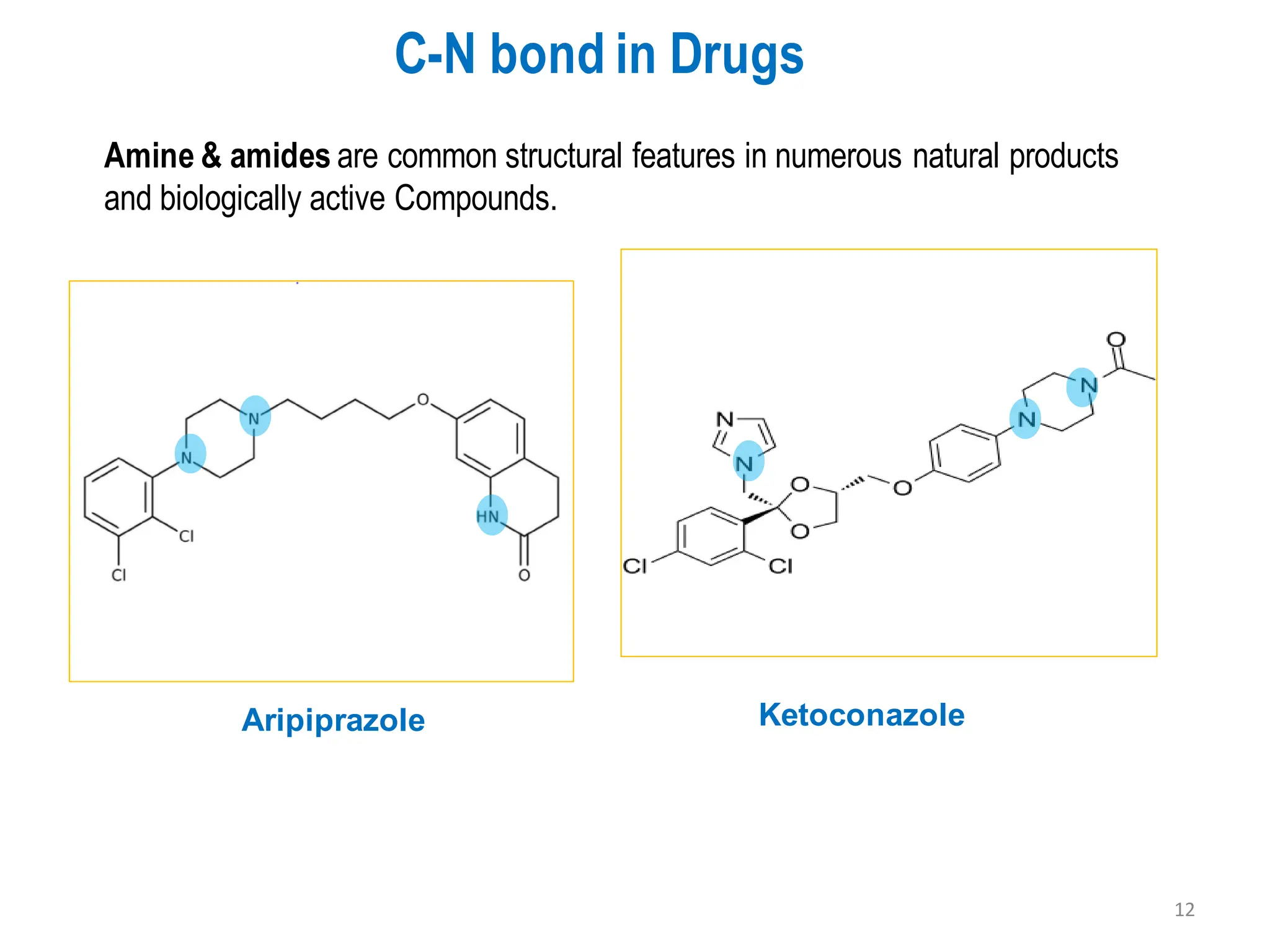
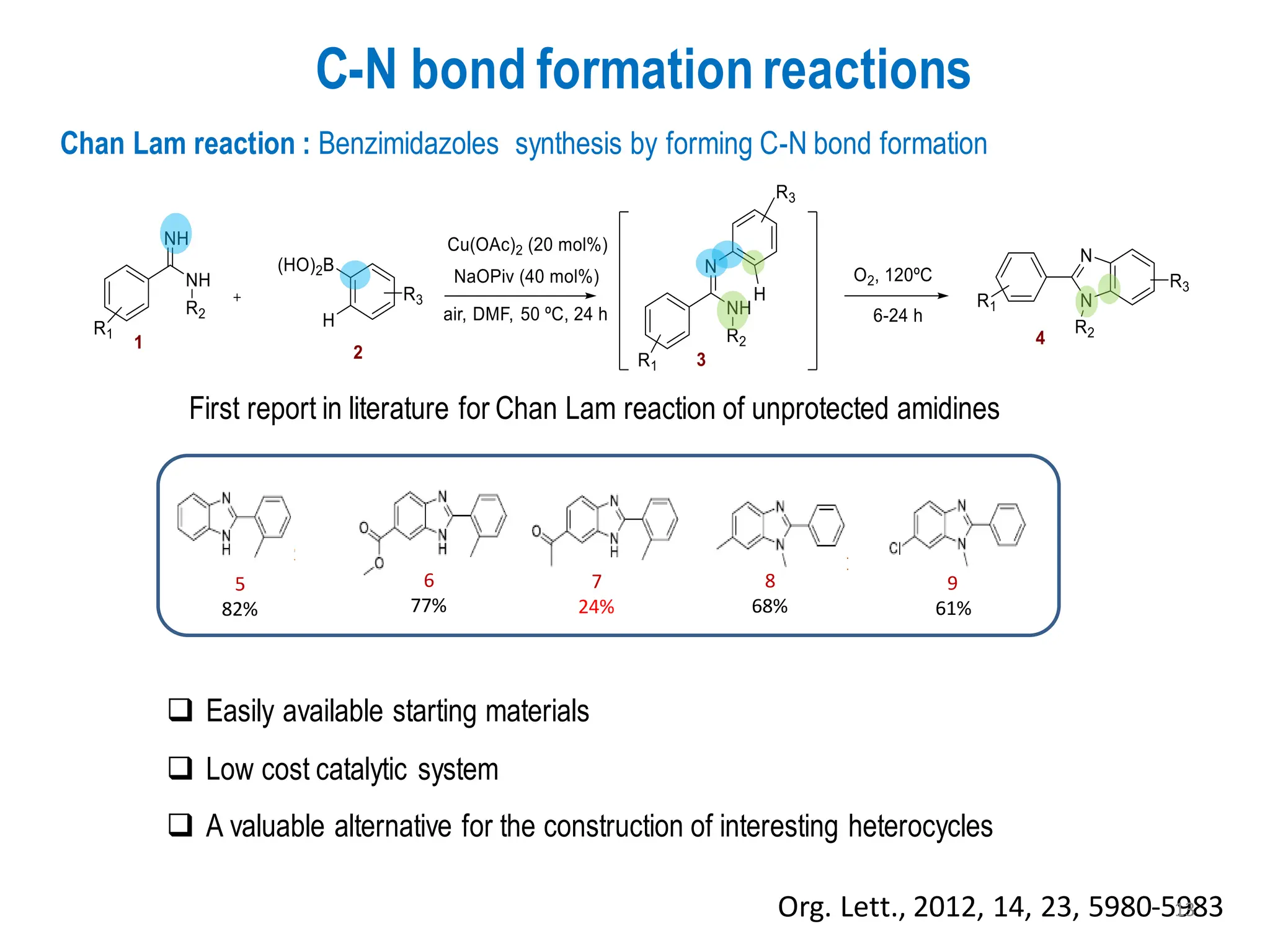
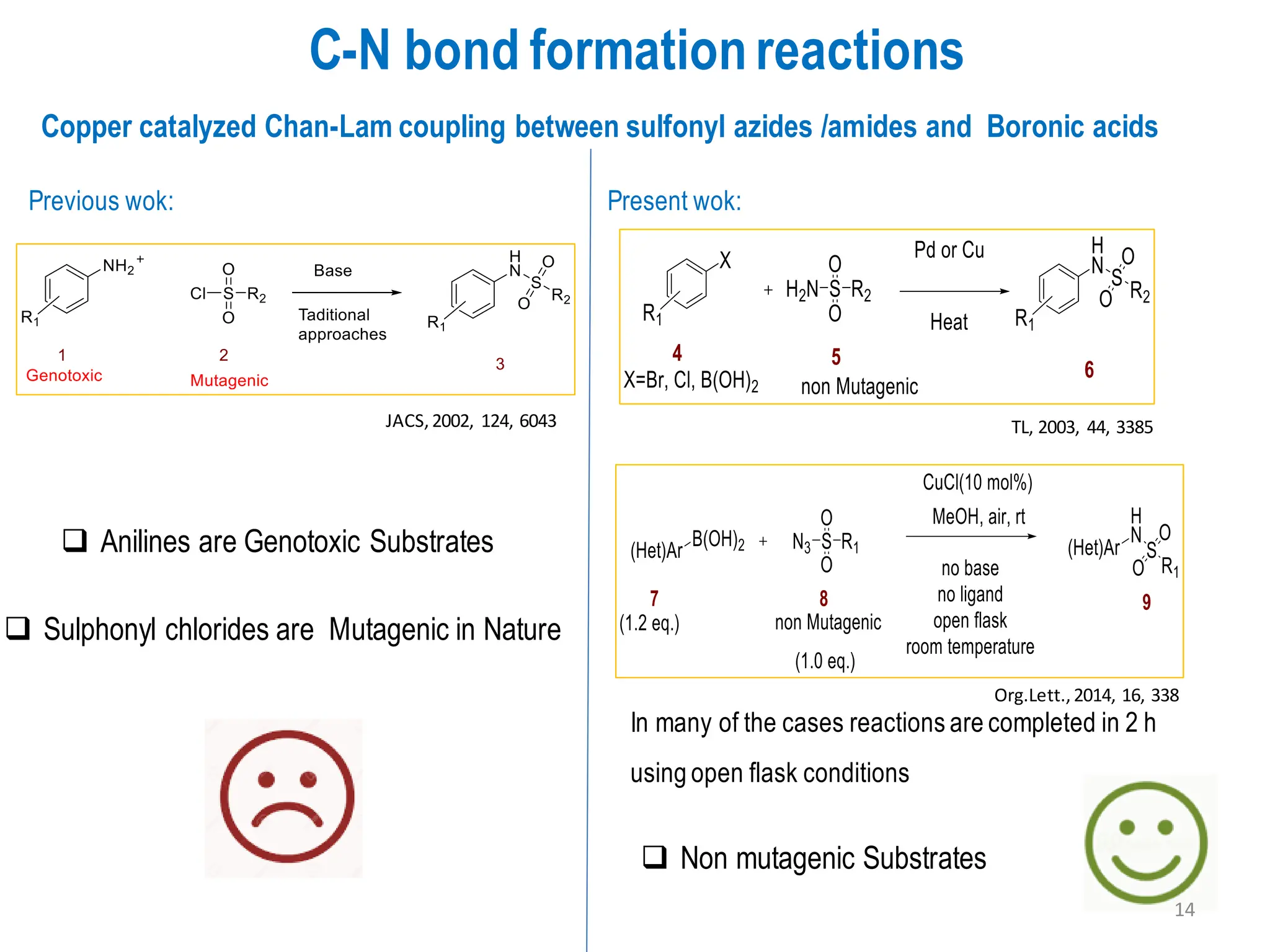
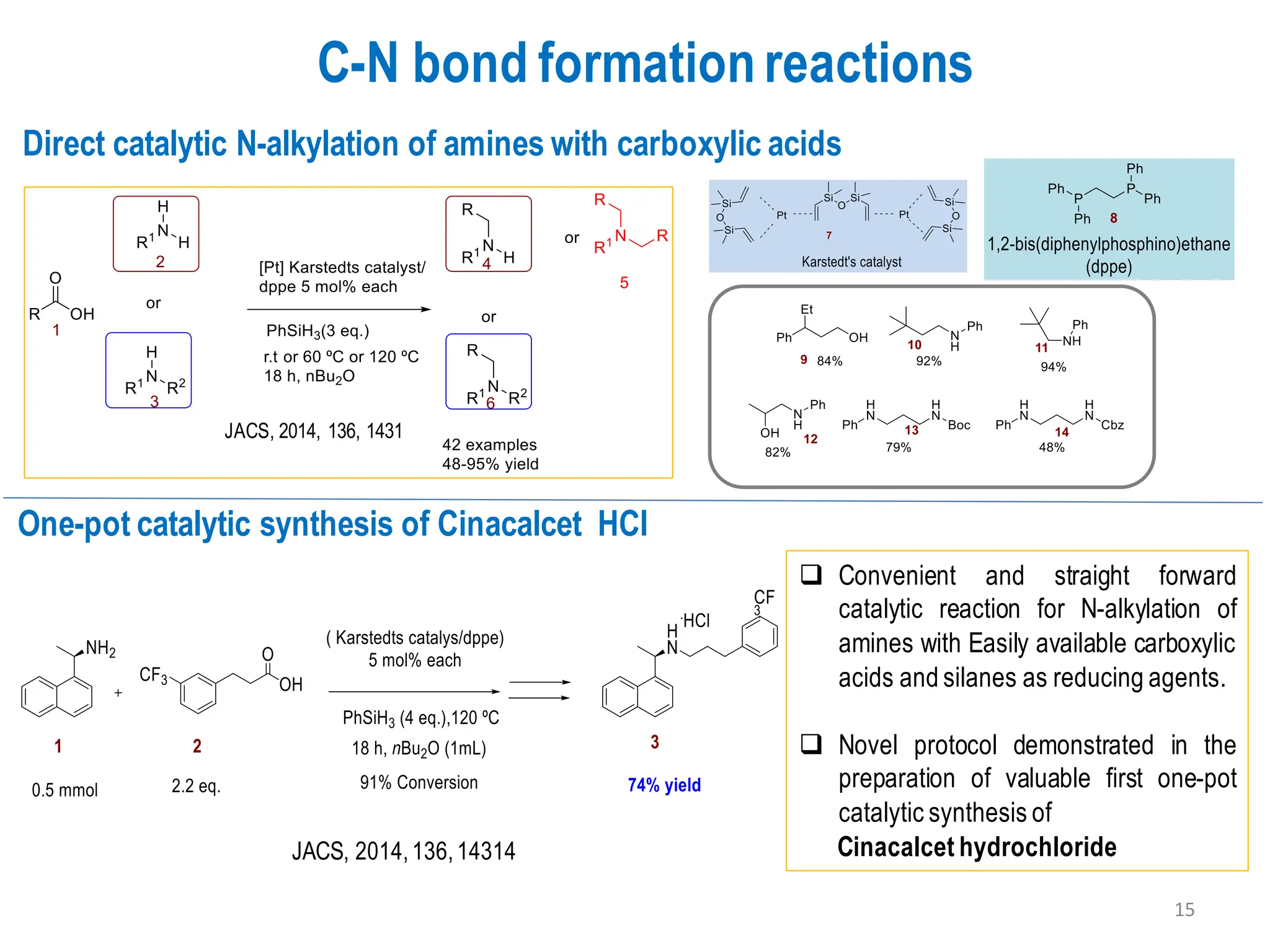
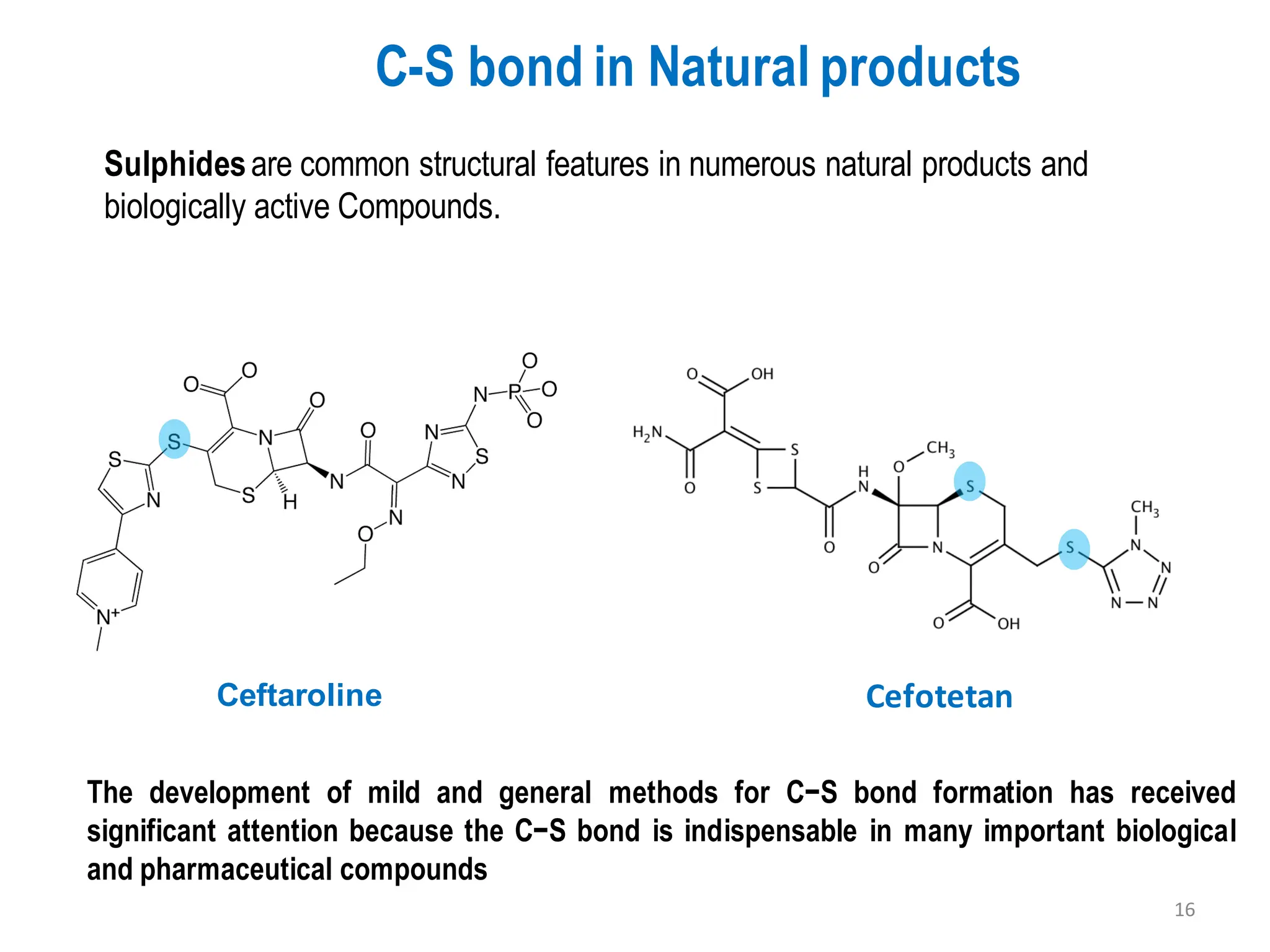
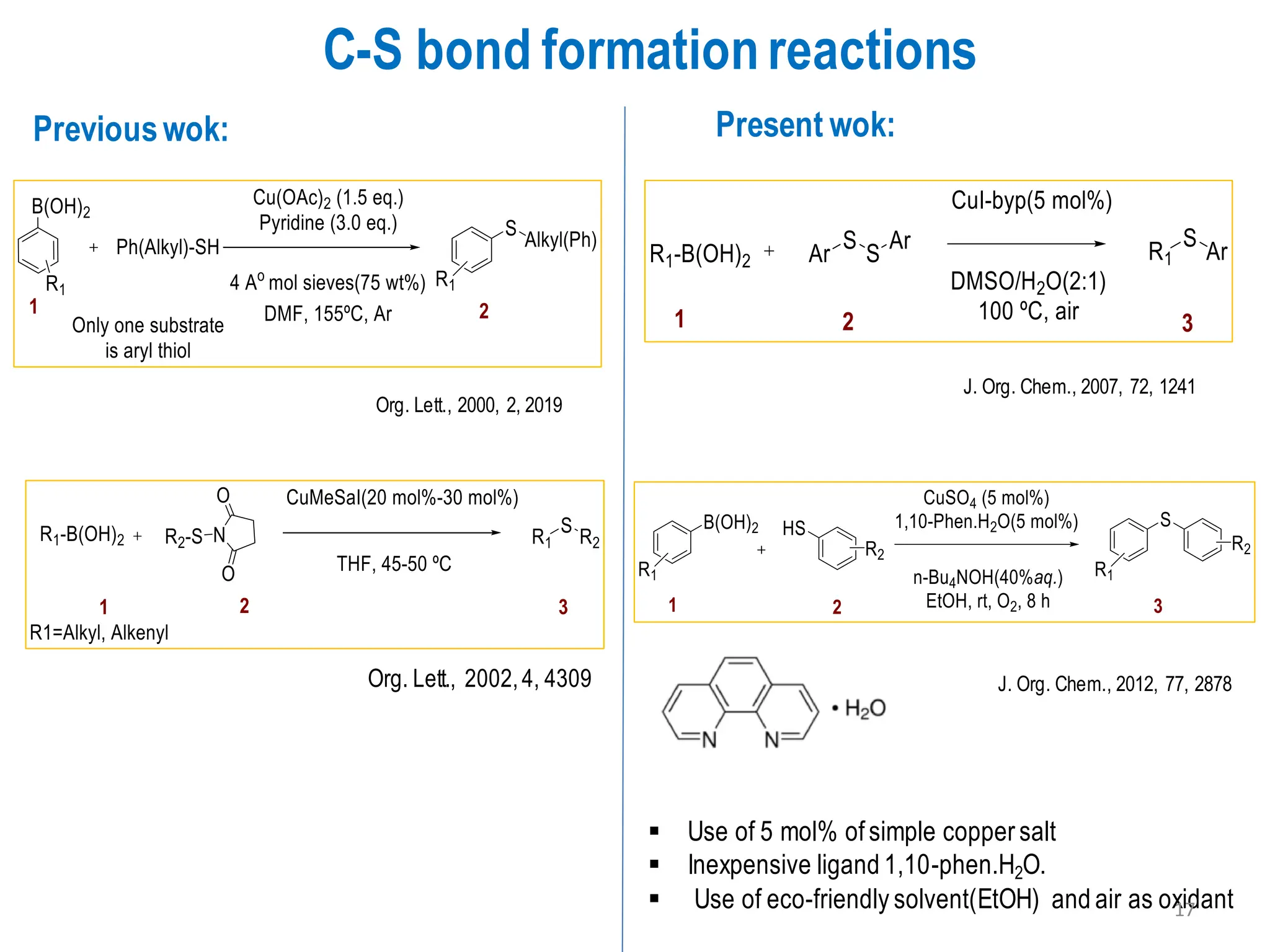
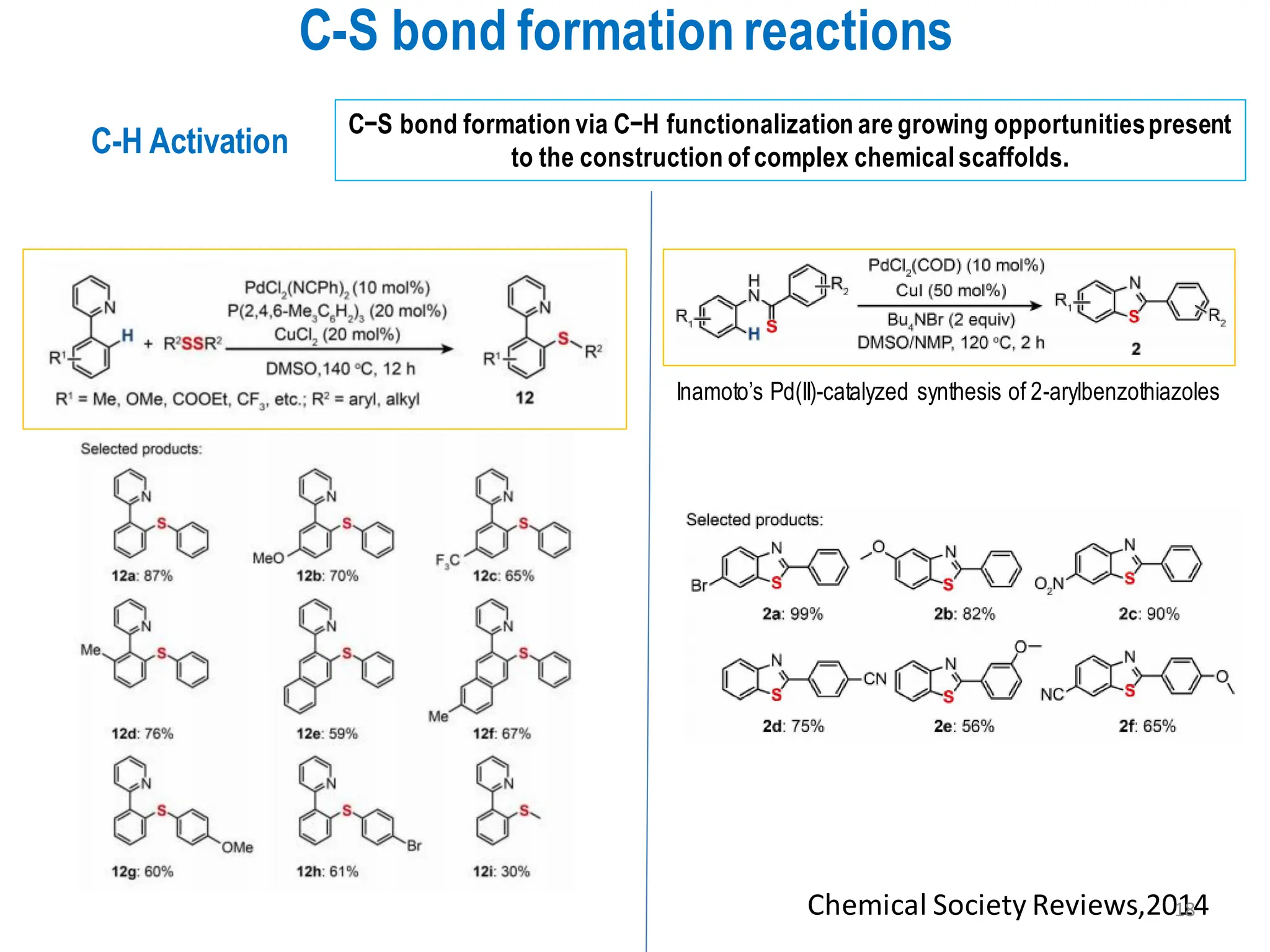
The presentation "C-O, C-N and C-S Bond Formation Methods" delves into the pivotal role of carbon-heteroatom (C-O, C-N, C-S) bond formation in synthetic organic and medicinal chemistry, focusing on their significance in natural products and pharmaceuticals. It surveys key synthetic strategies, from historical methods like Williamson ether synthesis to advanced transition metal-catalyzed reactions such as Chan-Lam and Buchwald-Hartwig couplings, emphasizing their efficiency and practical applications. The discussion highlights the importance of these bonds in drug-receptor interactions and molecular synthesis, while addressing challenges and recent innovations in eco-friendly, cost-effective catalytic systems. The presentation concludes by affirming the enduring value of these reactions as essential tools for synthesizing diverse, critical compounds across agrochemical, pharmaceutical, and fine chemical industries.