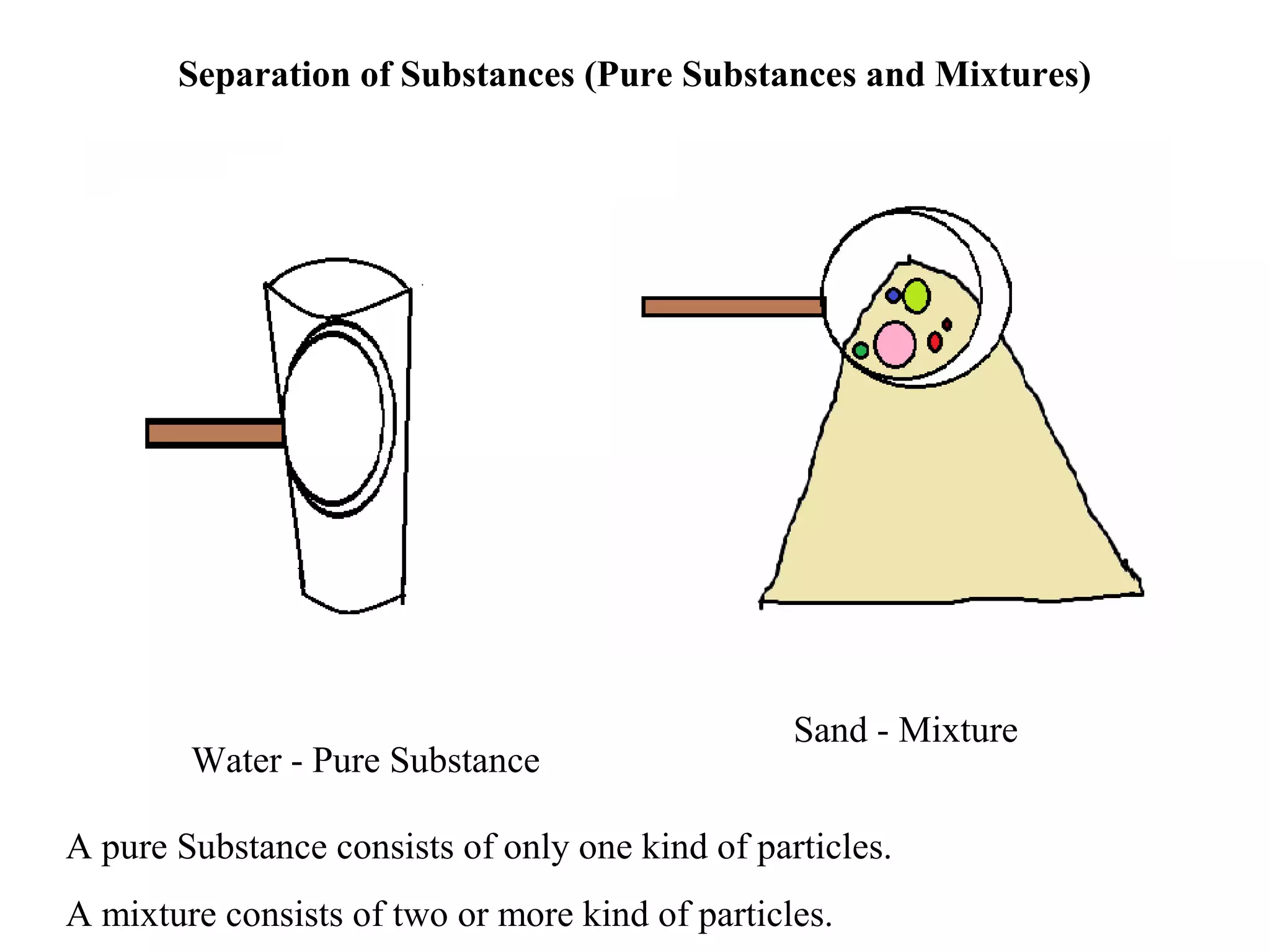
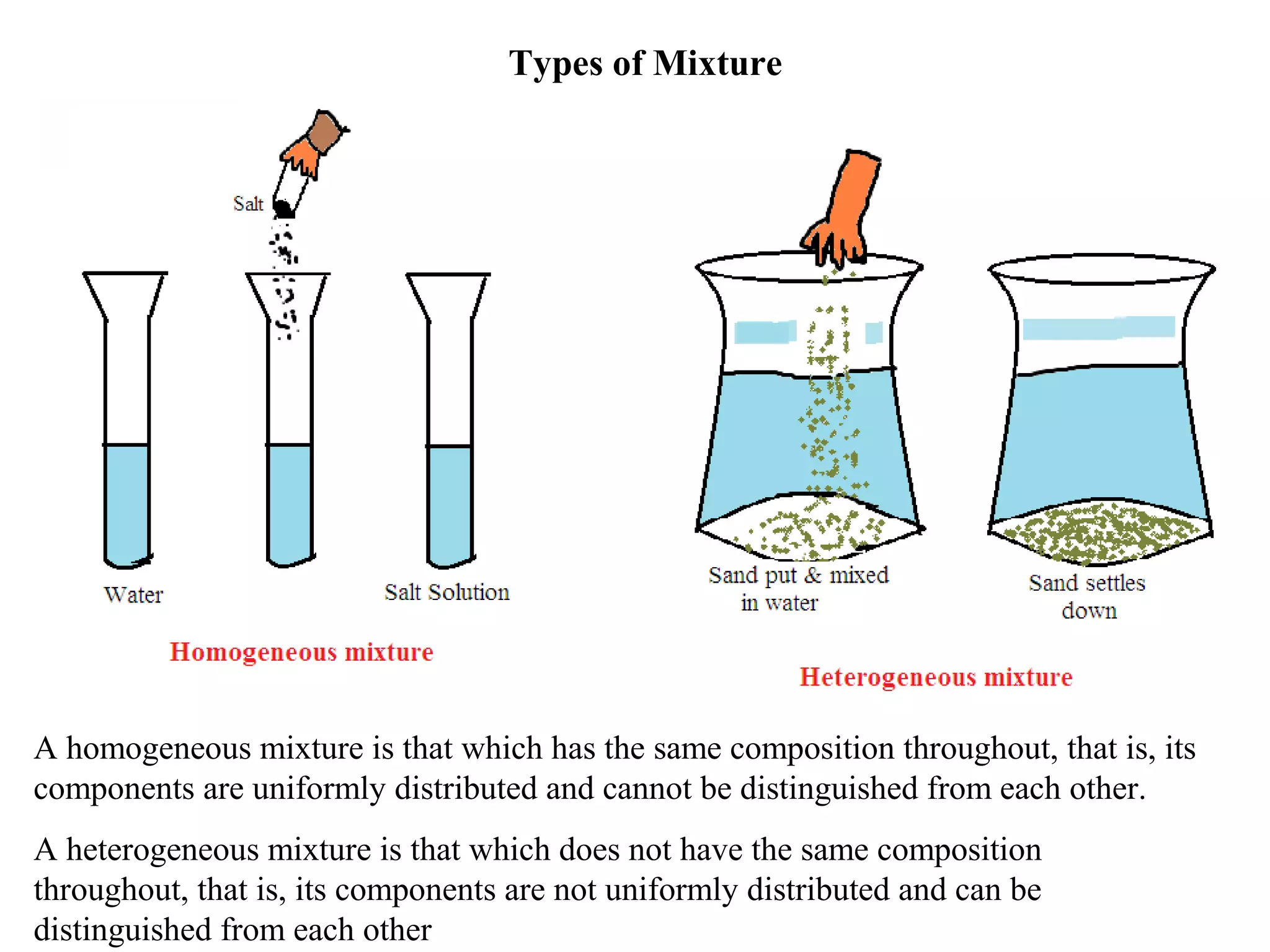
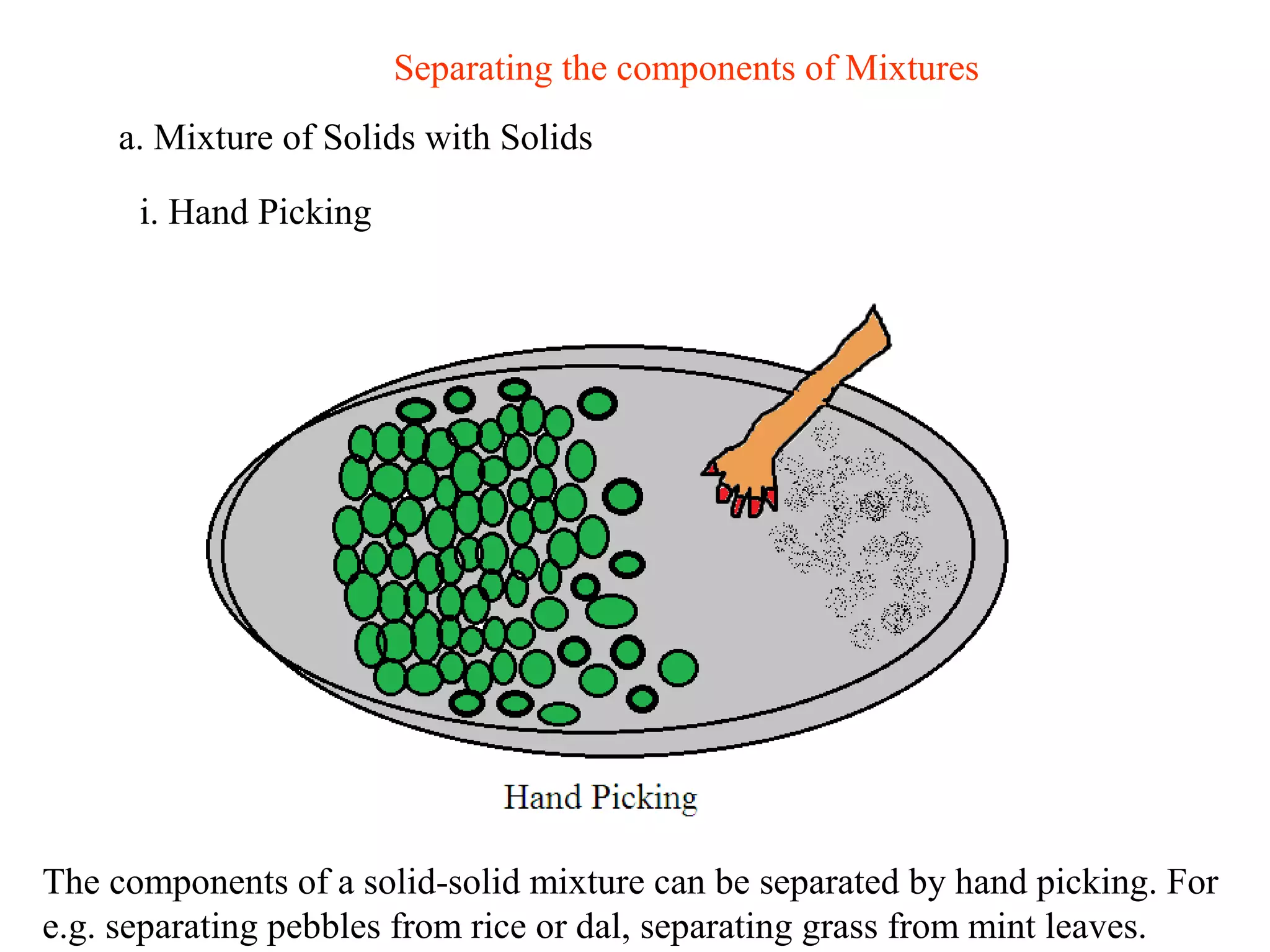
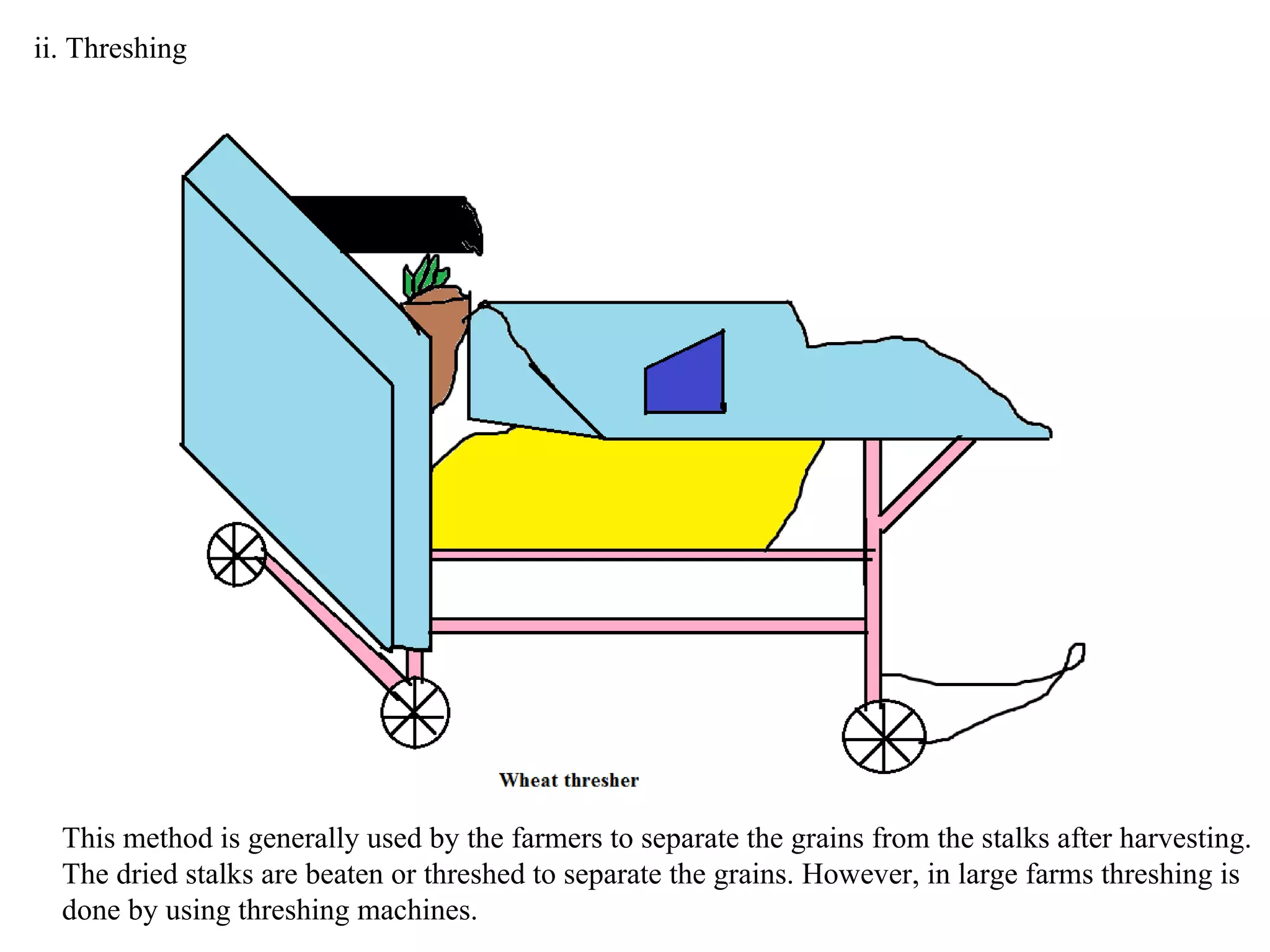
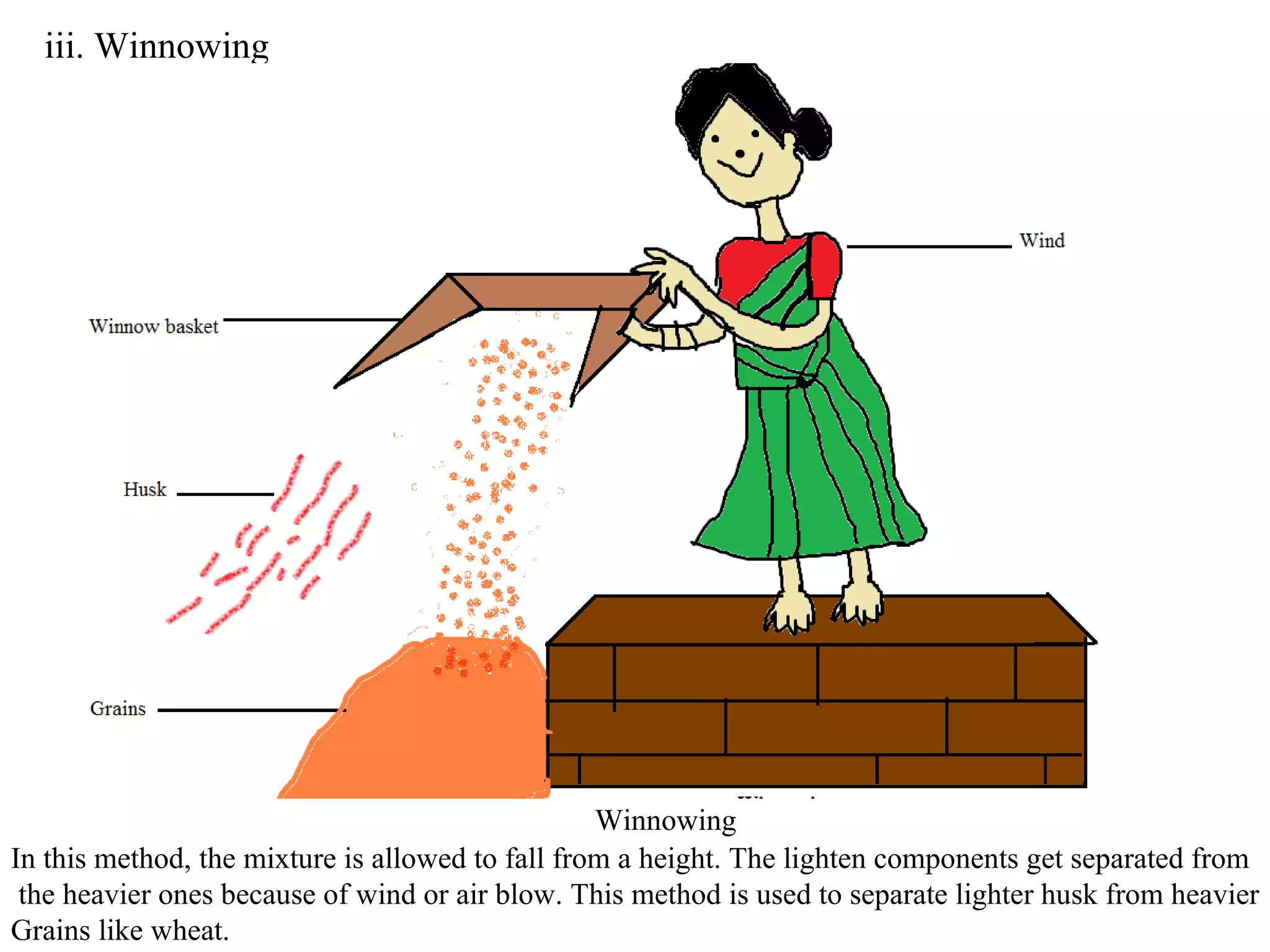
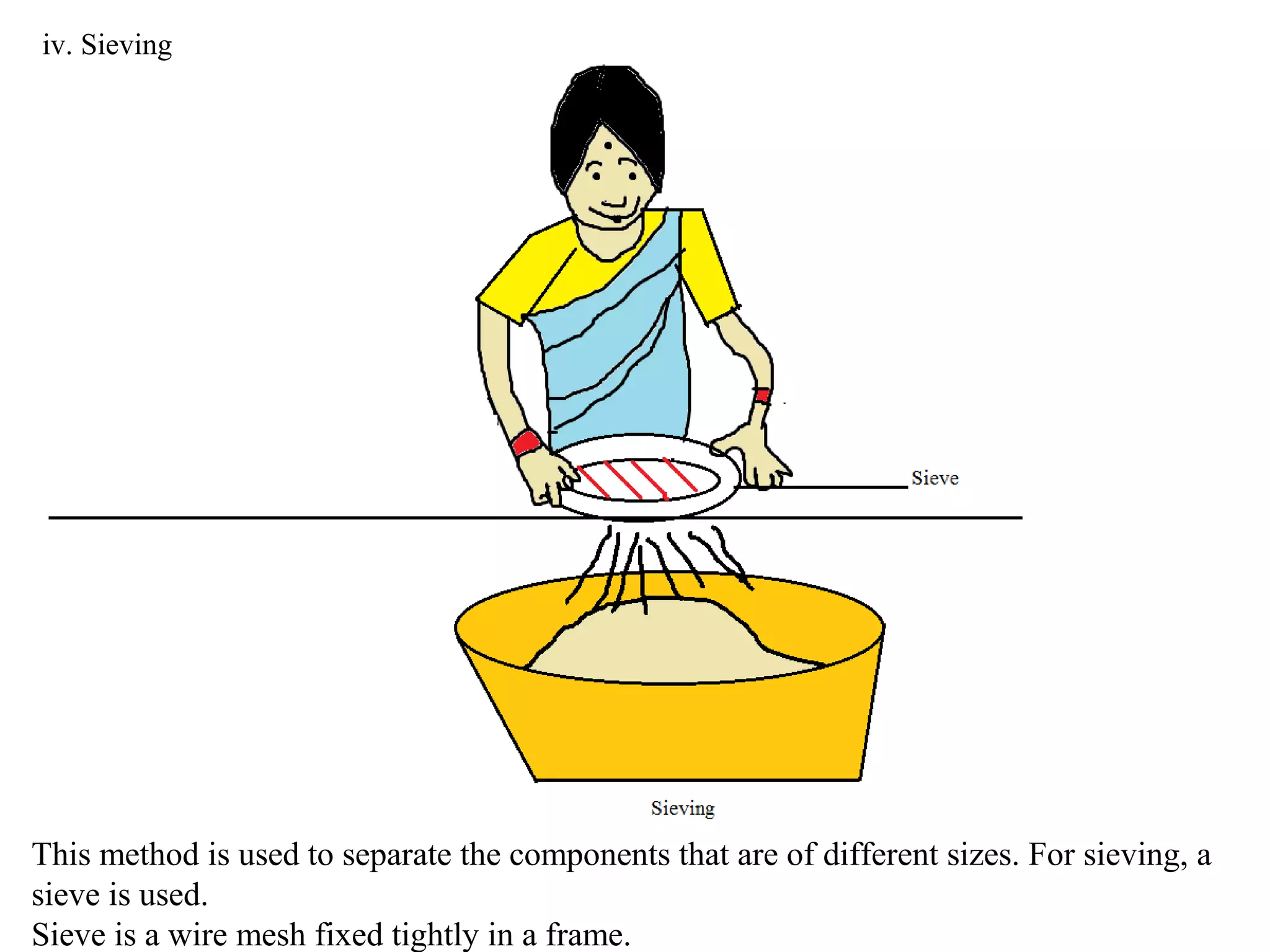
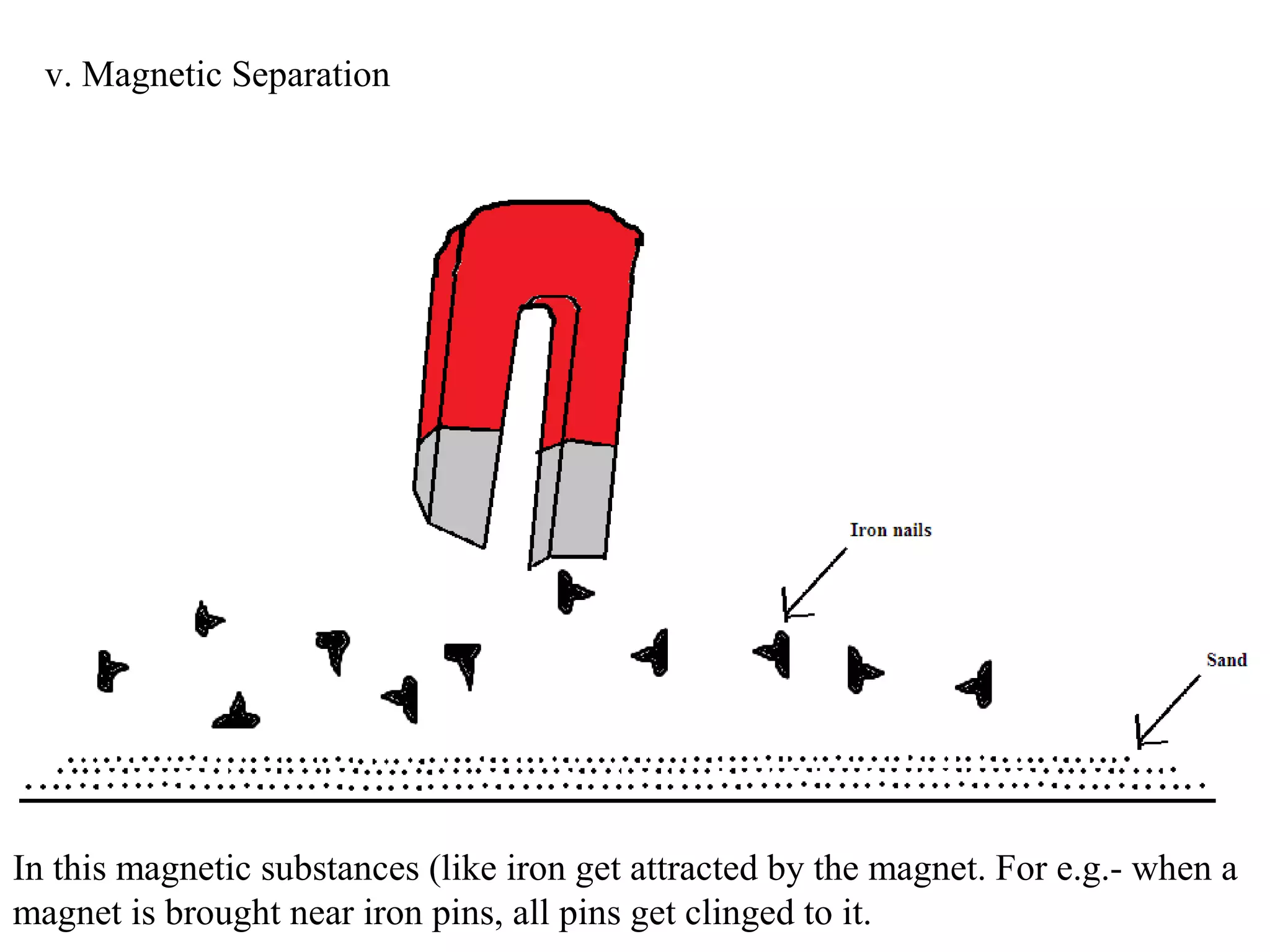
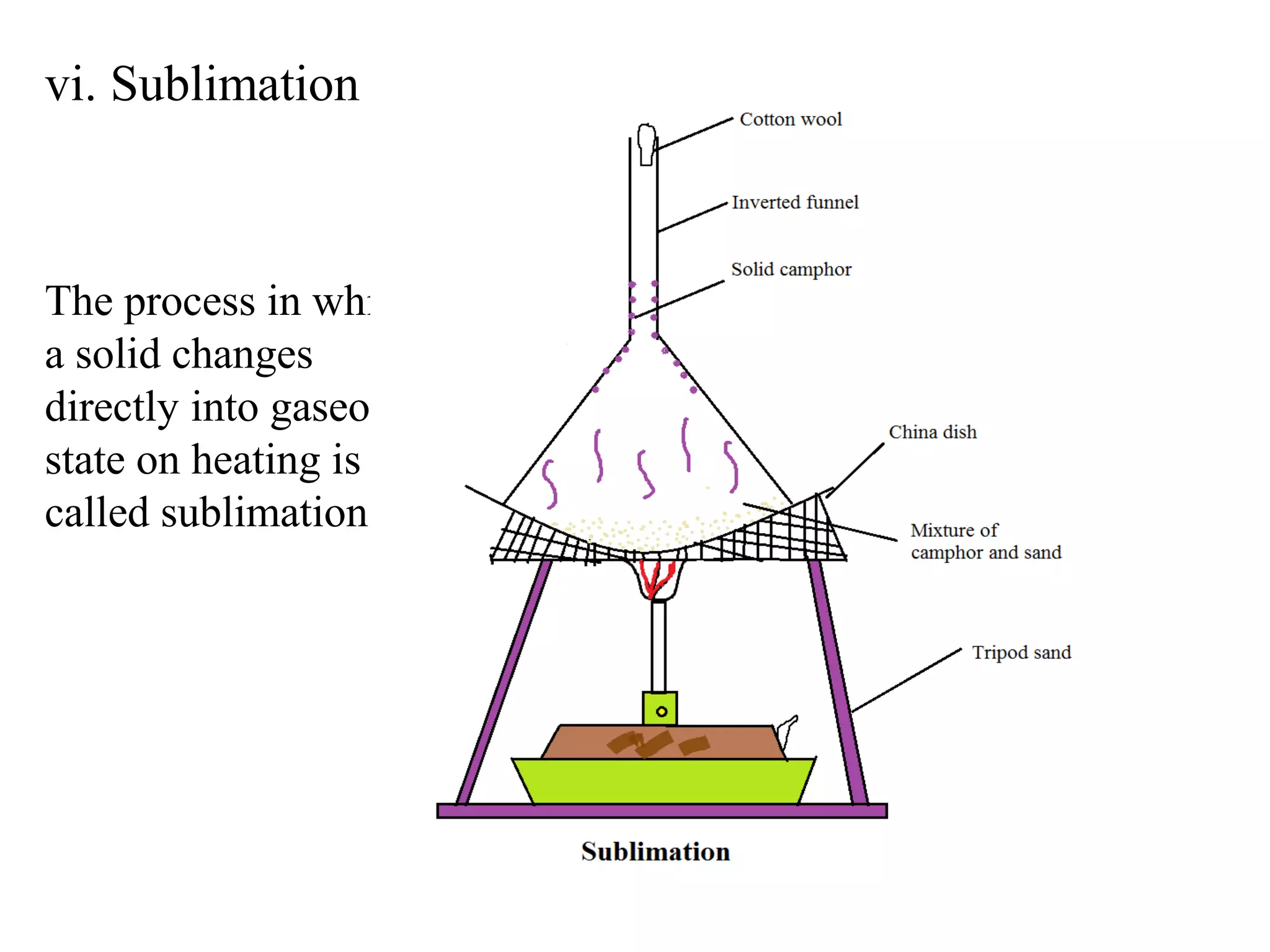
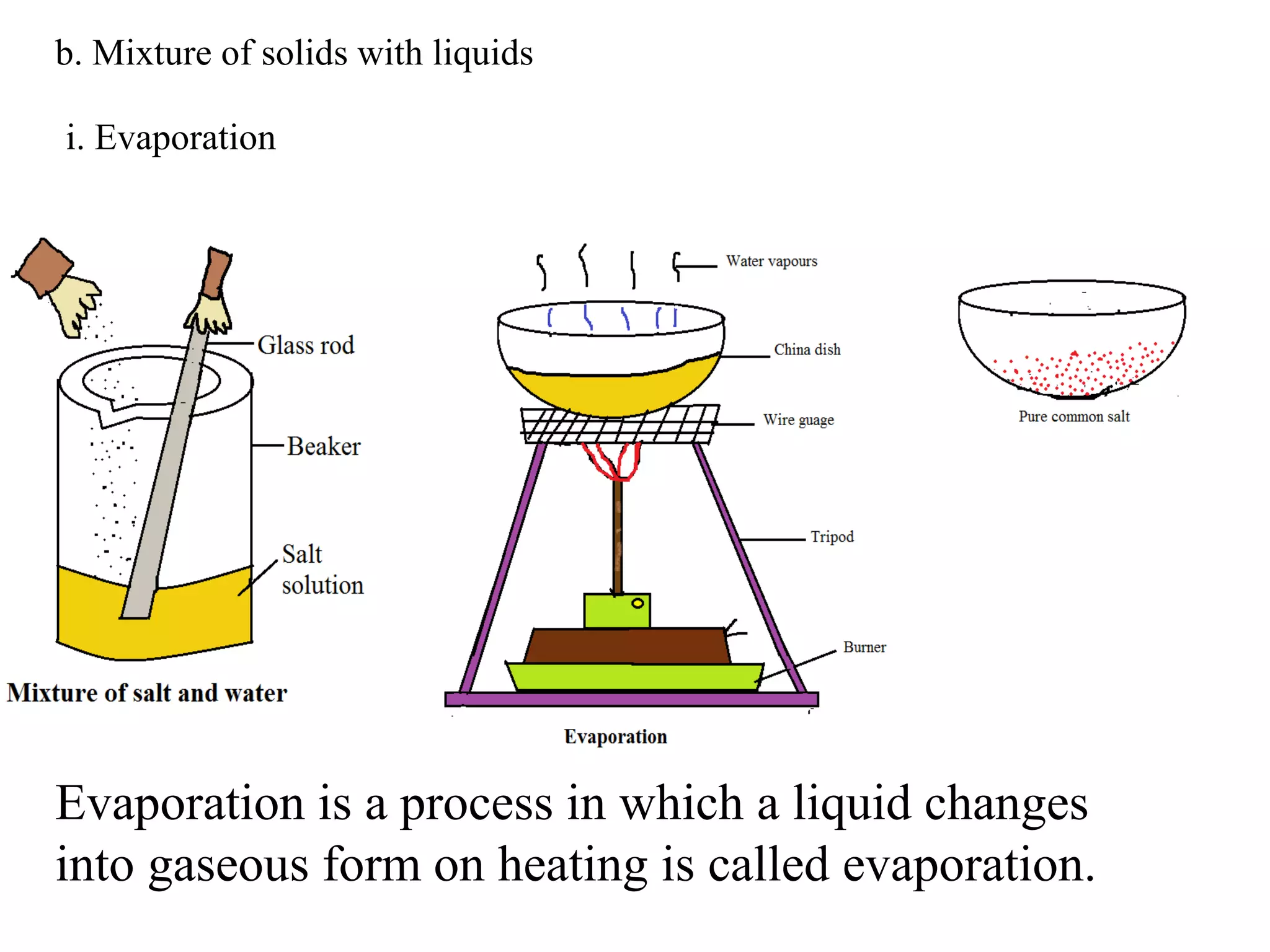
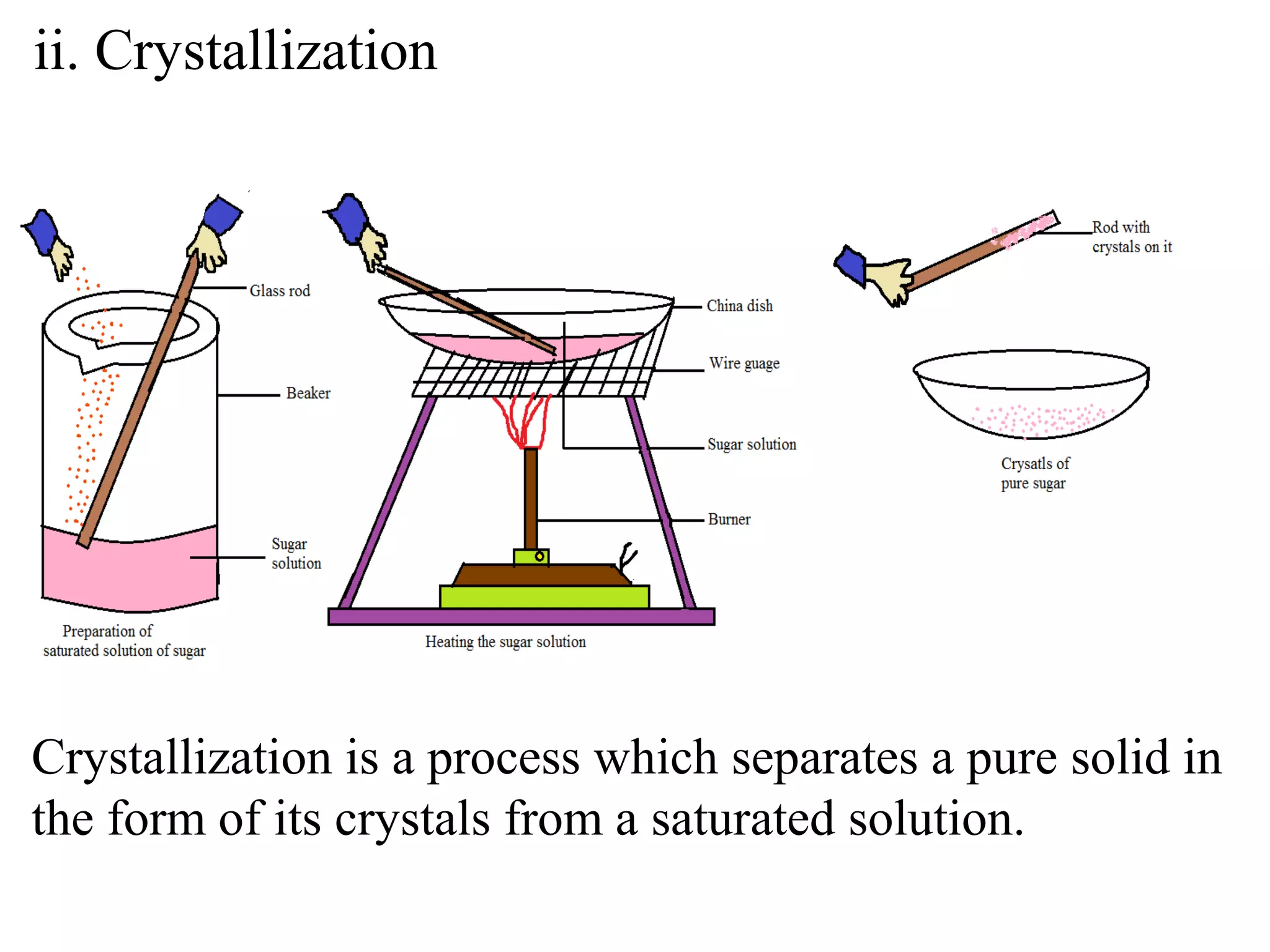
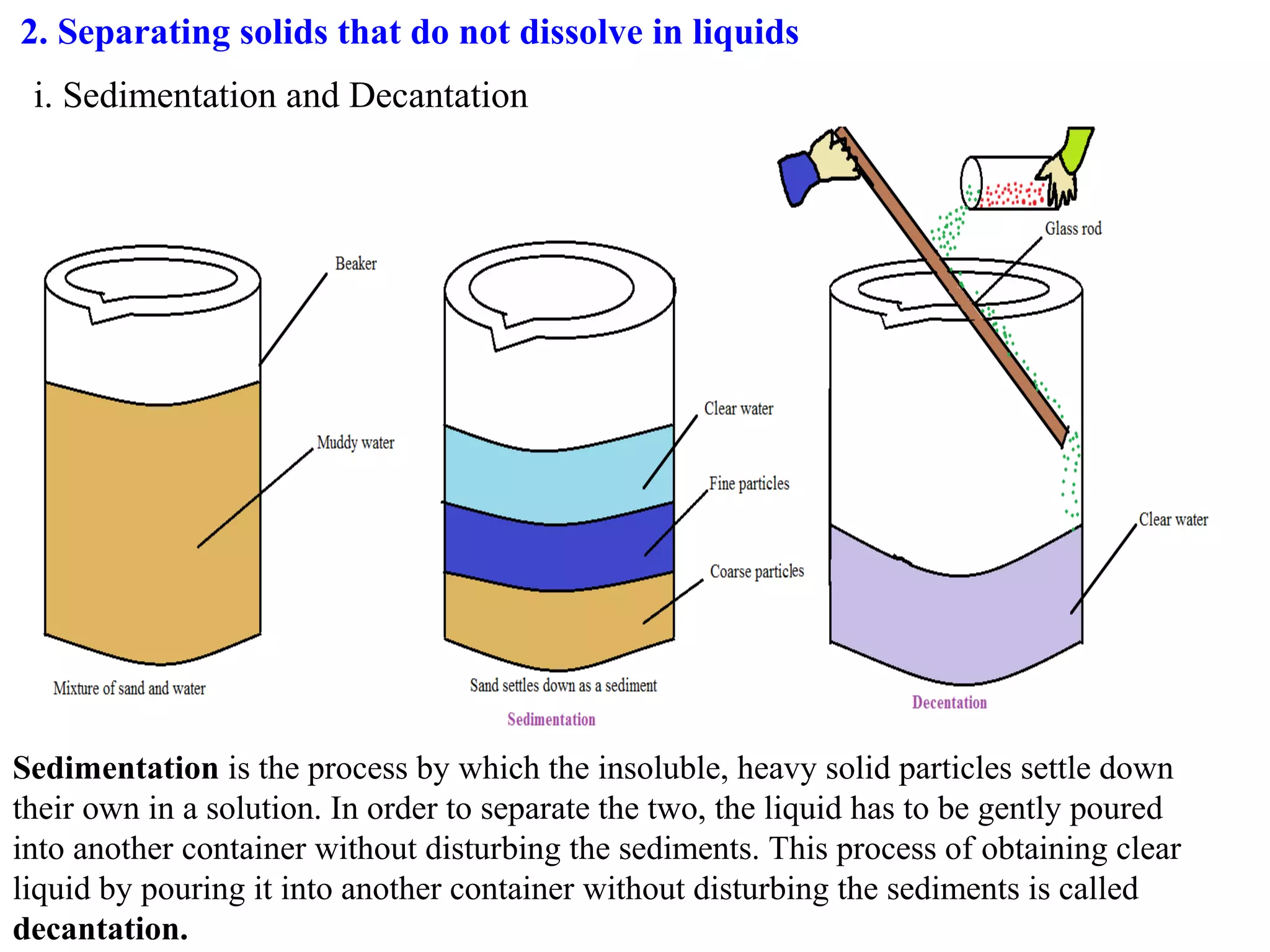
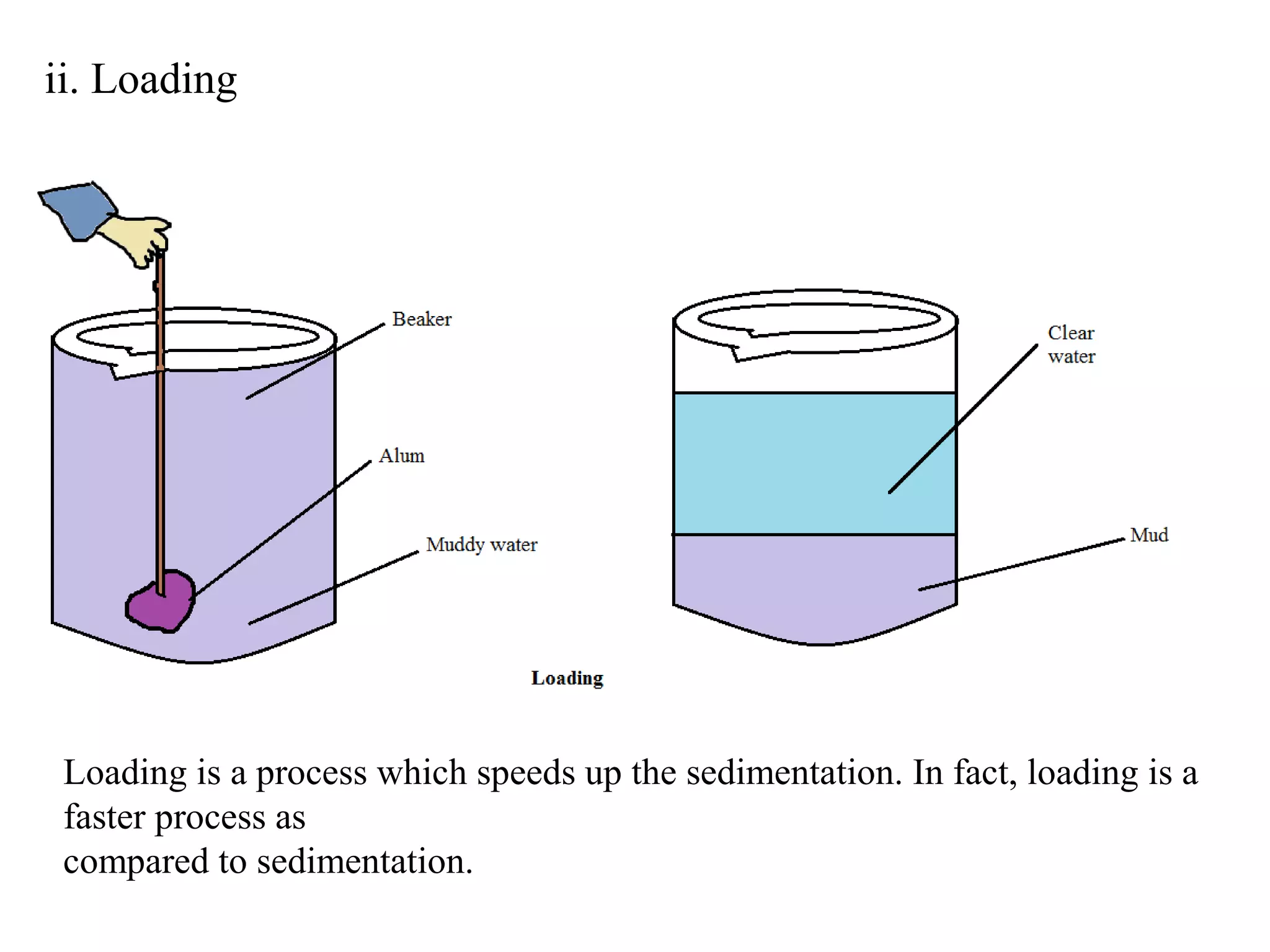
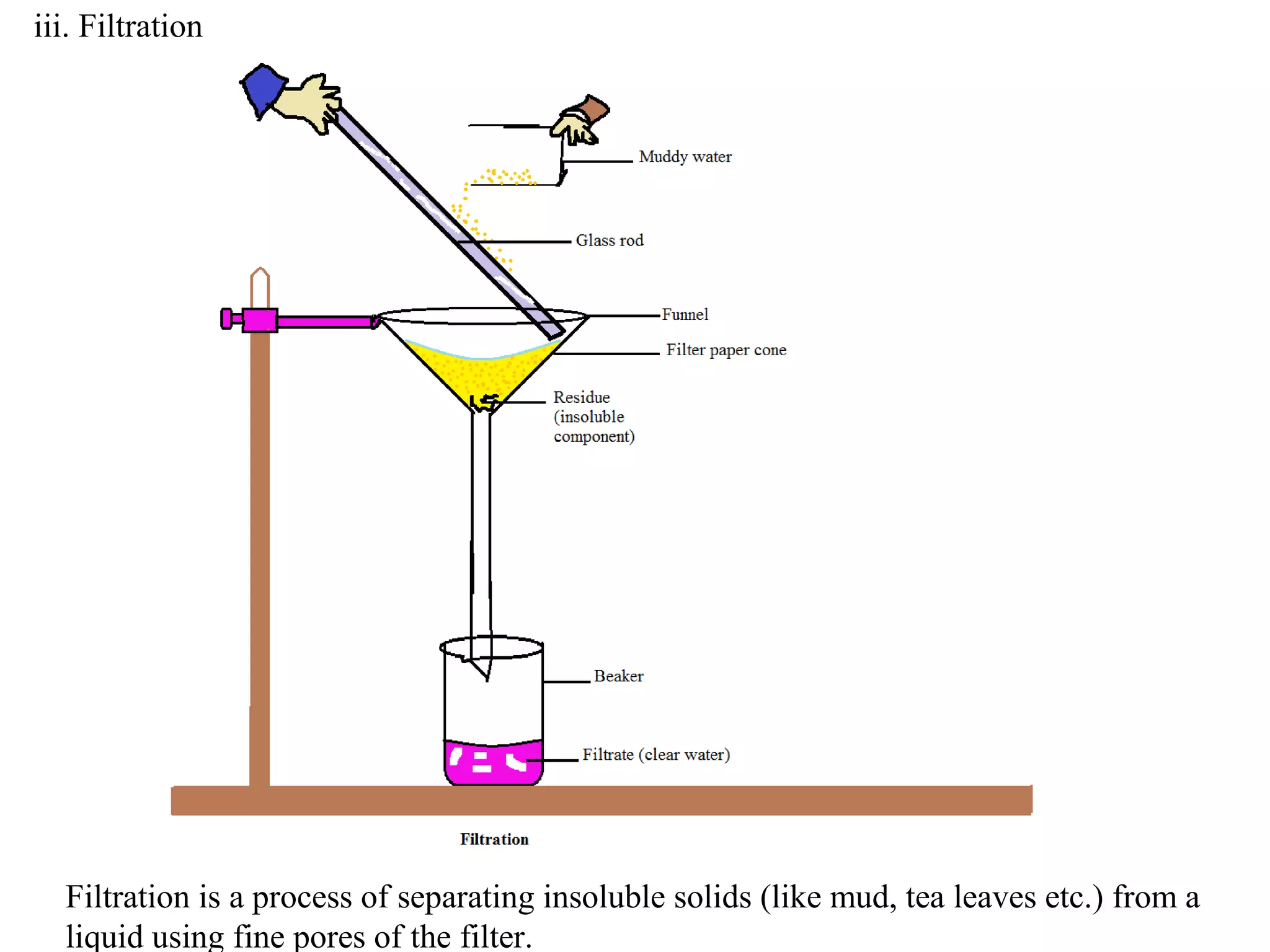
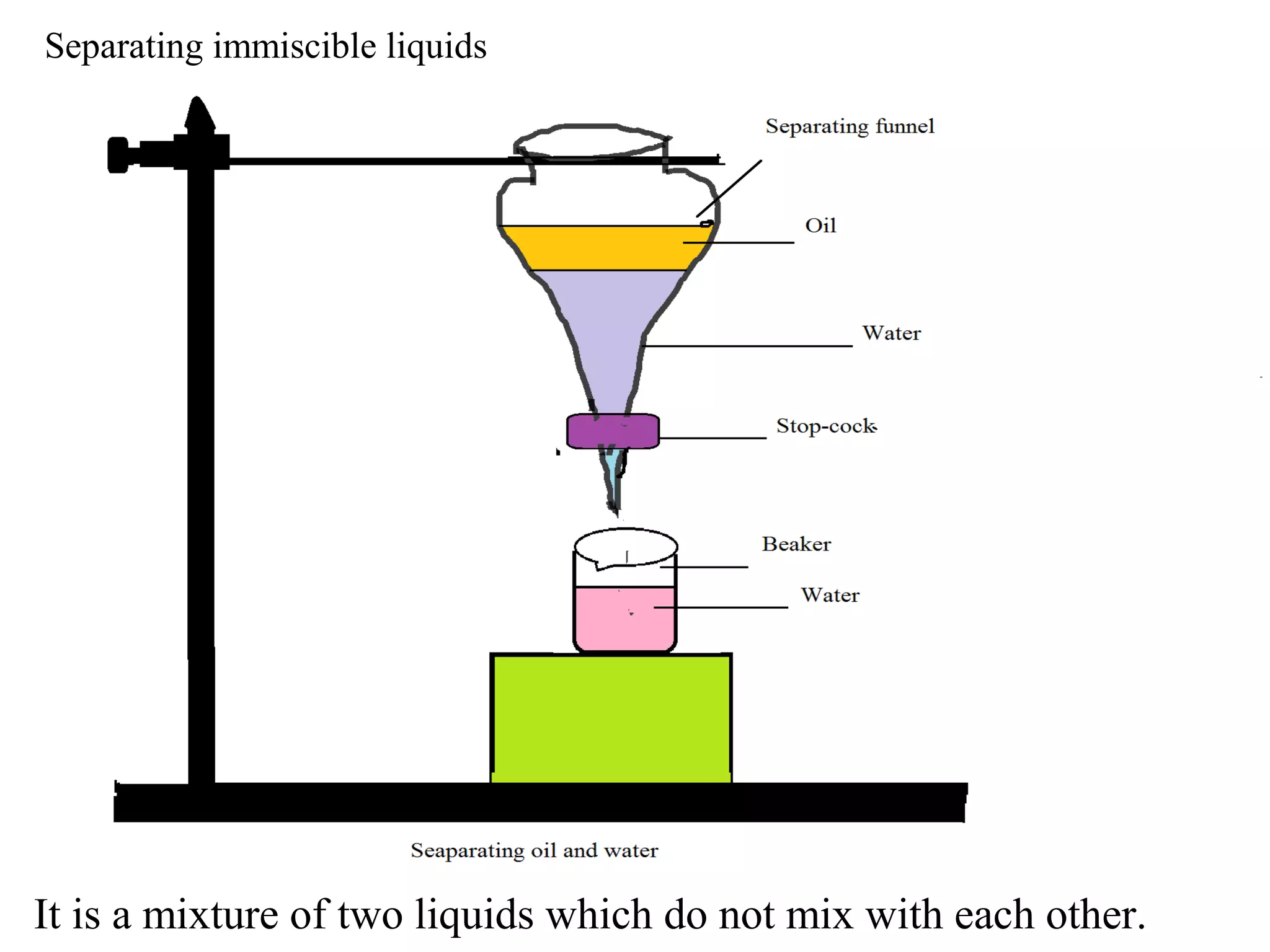
A mixture consists of two or more substances that are not chemically combined. There are several methods to separate the components of mixtures.
[1] For solid-solid mixtures, components can be separated by hand picking, threshing, winnowing, sieving, magnetic separation, or sublimation. [2] For solid-liquid mixtures, evaporation or crystallization can be used. [3] Other methods include sedimentation, decantation, loading, and filtration to separate solids from liquids. Immiscible liquids can also be separated since they do not mix.