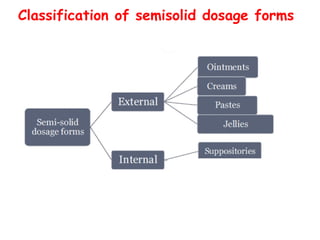
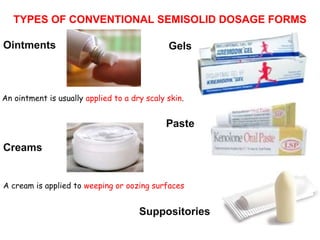
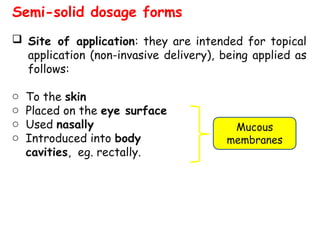
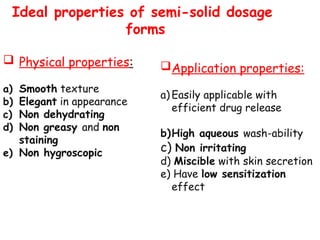
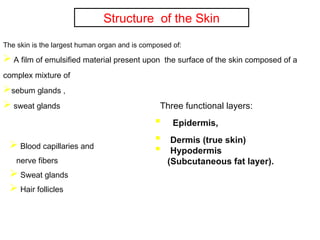
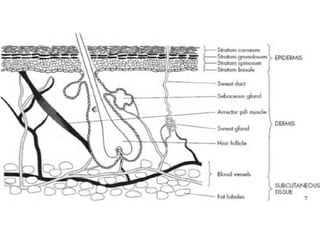
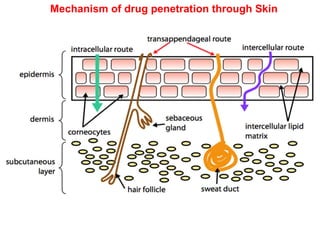
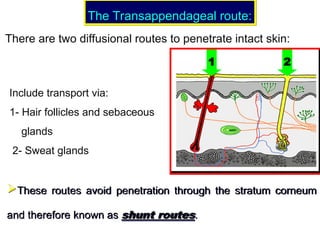
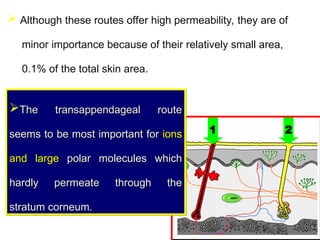
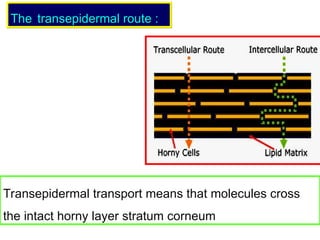
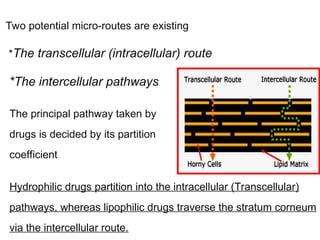
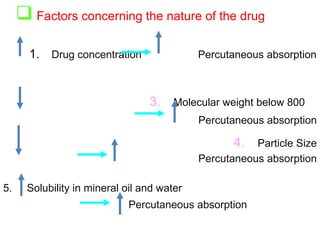
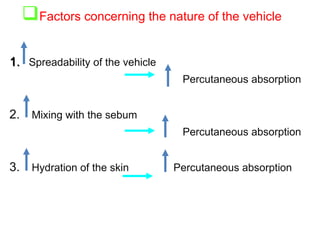
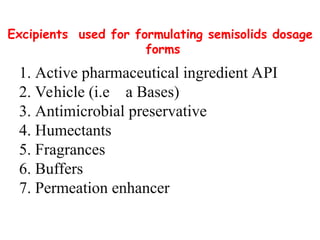
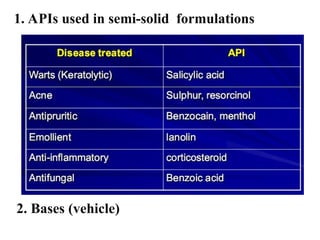
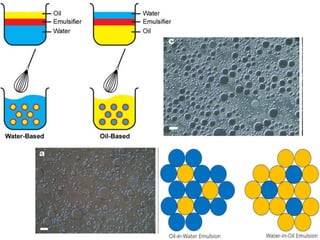
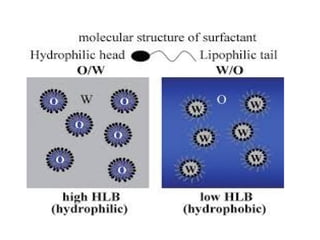
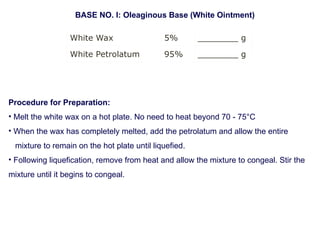
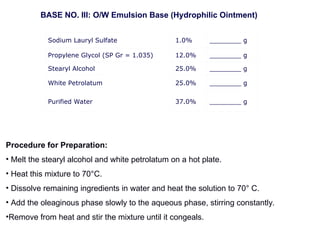
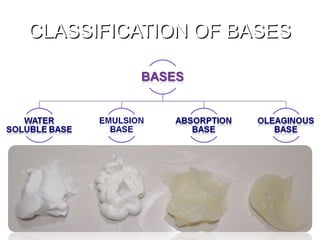
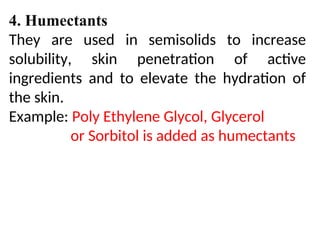
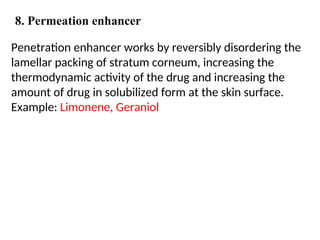
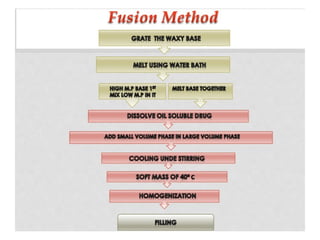
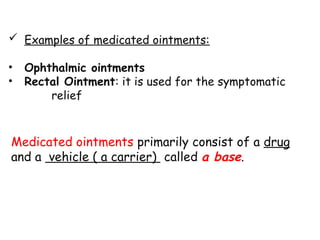
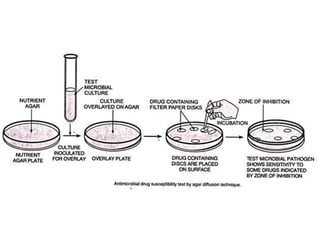
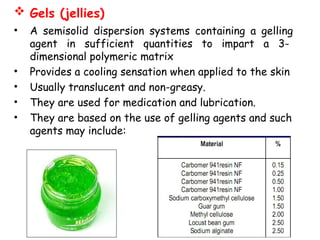
The document provides an overview of semi-solid dosage forms, highlighting their therapeutic or protective applications and classification into various types such as ointments, creams, gels, and pastes. It discusses the ideal properties of these forms, their advantages and disadvantages, routes of skin penetration, and factors affecting drug absorption through the skin. Additionally, it elaborates on the composition and preparation methods for different bases used in semi-solid formulations, including oleaginous, absorption, water-removable, and water-soluble bases.