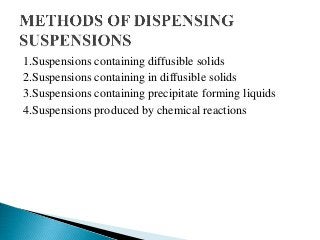
This document discusses pharmaceutical suspensions. It defines suspensions as biphasic liquid dosage forms containing finely divided solid particles dispersed in a liquid or semisolid vehicle. Suspensions can be administered orally, parenterally, for ophthalmic or external use. The document outlines the key properties suspensions must have and different types of additives that can be included. It also describes common methods for preparing different types of suspensions and tests used to evaluate suspension stability.