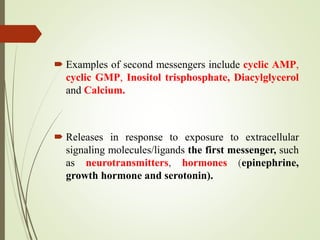
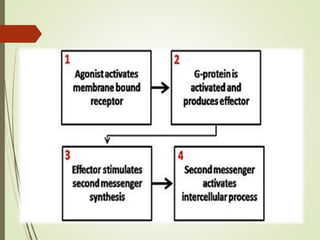
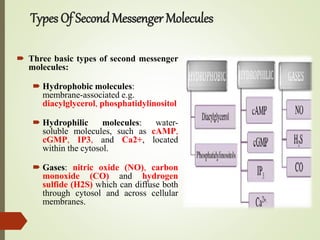
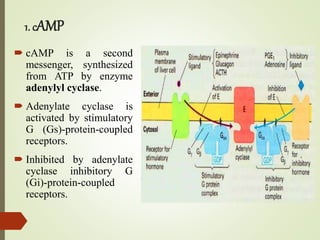
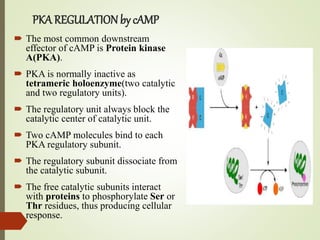
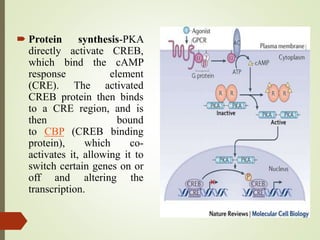
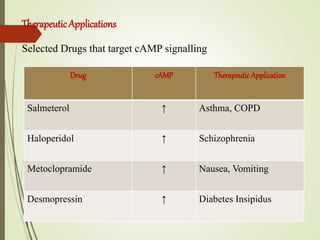
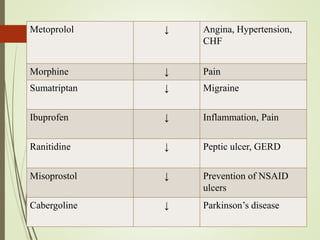
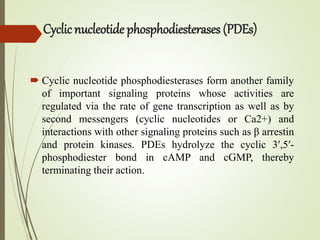
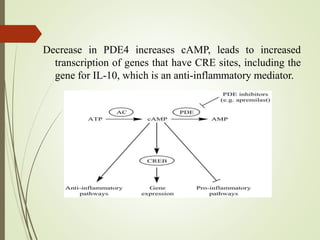
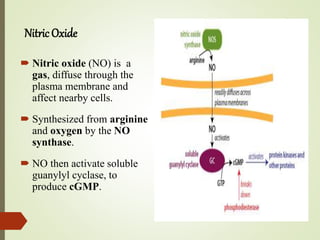
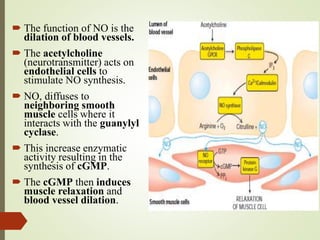
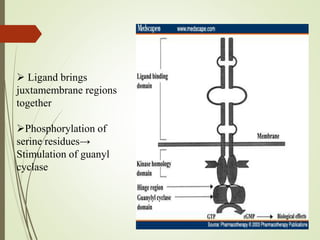
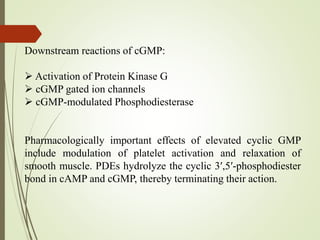
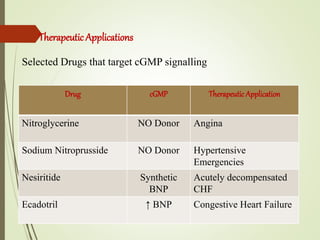
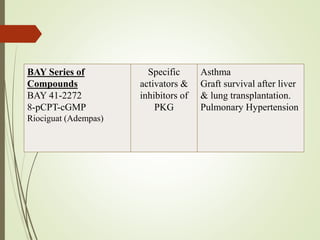
Second messengers are intracellular signaling molecules that are released within cells in response to extracellular first messengers like hormones and neurotransmitters. They amplify and propagate intracellular signals. Examples include cyclic AMP (cAMP), cyclic GMP (cGMP), inositol trisphosphate, and calcium. cAMP and cGMP are produced from ATP and GTP by adenylate and guanylate cyclases, respectively. They activate downstream effector proteins like protein kinase A and G. This leads to phosphorylation of various target proteins and physiological responses like metabolism, gene expression, cell survival, proliferation and apoptosis. The document discusses the mechanisms, targets, functions and therapeutic applications of cAMP and cGMP second messenger systems in detail.