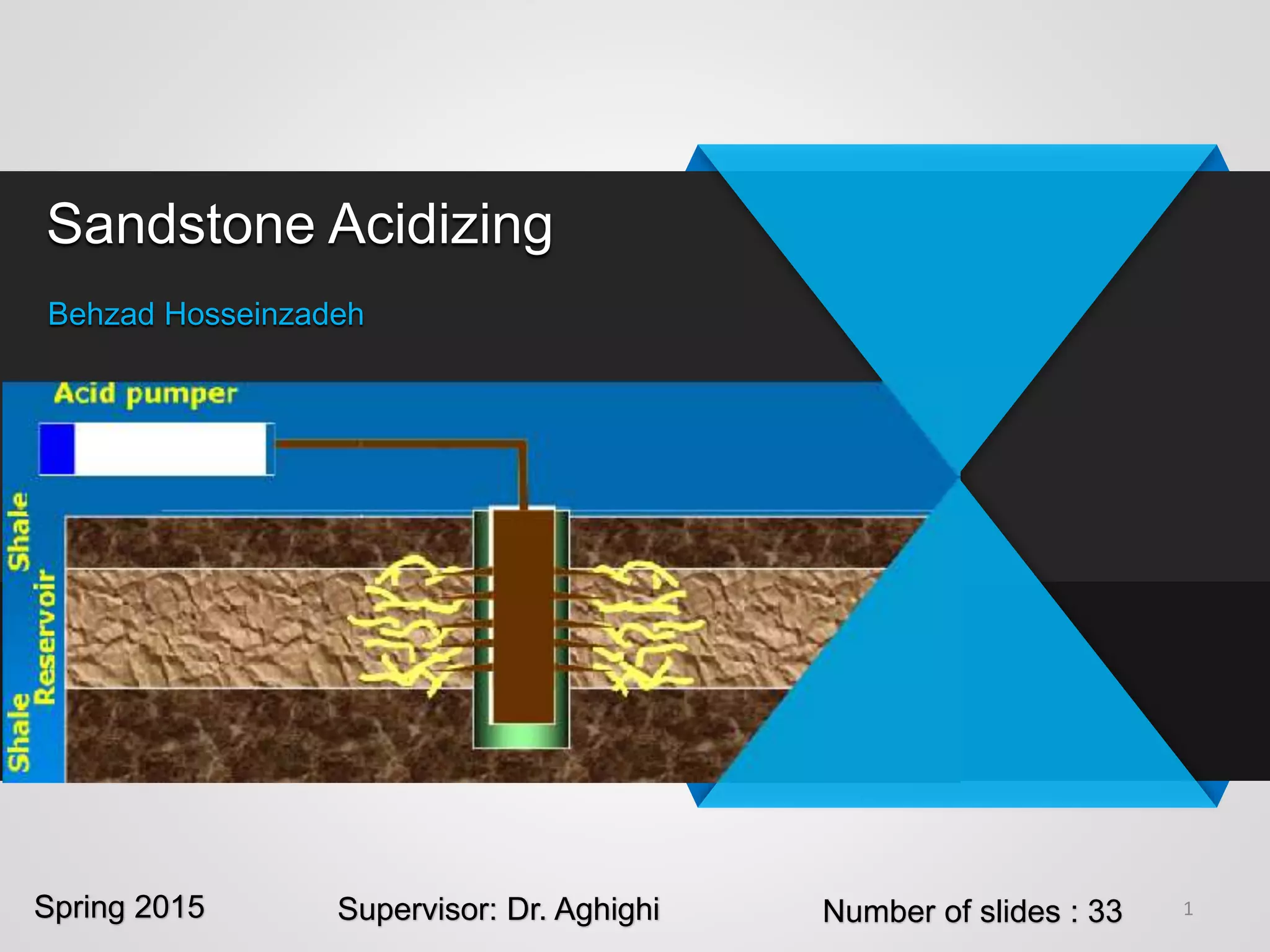
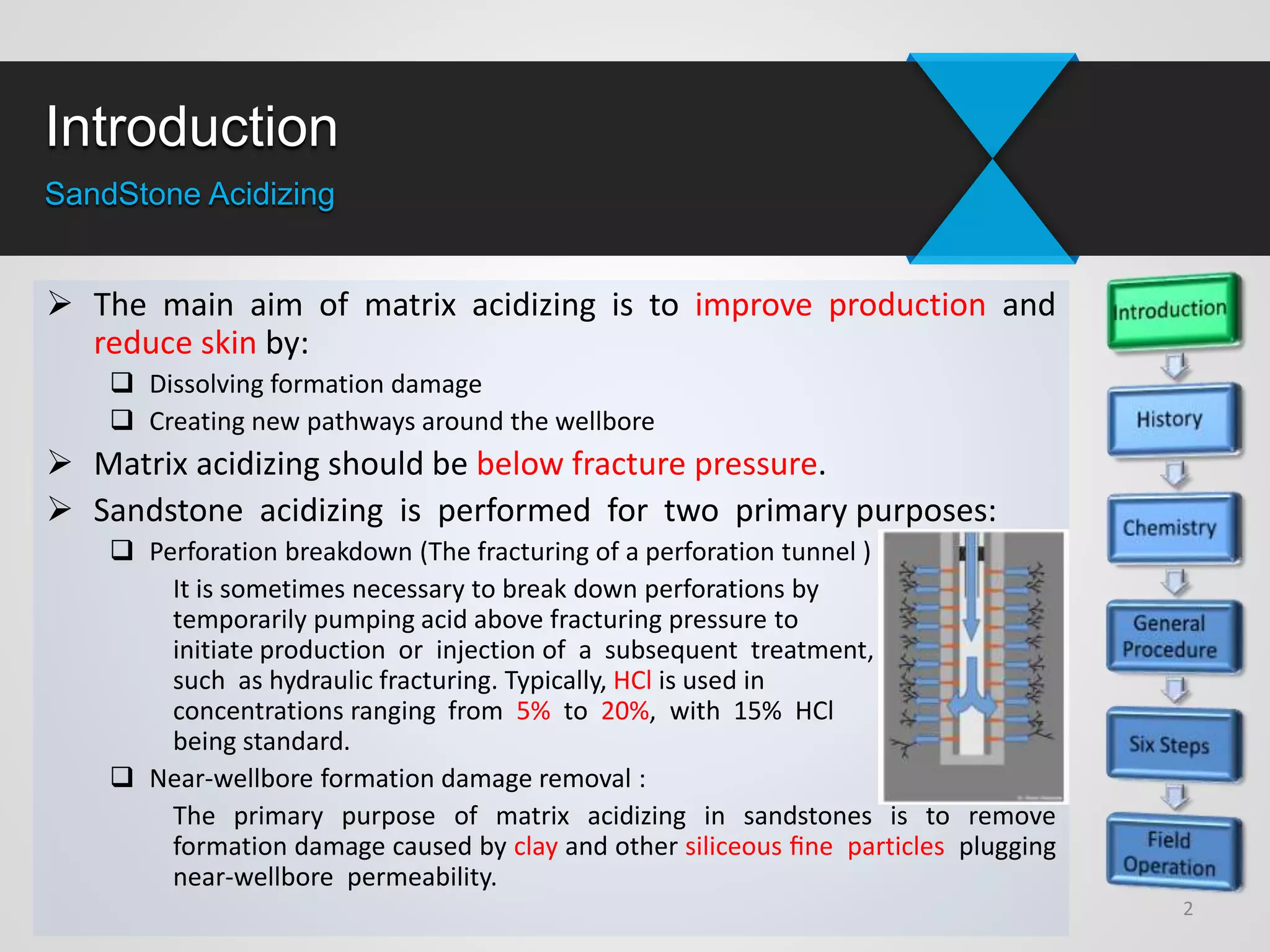
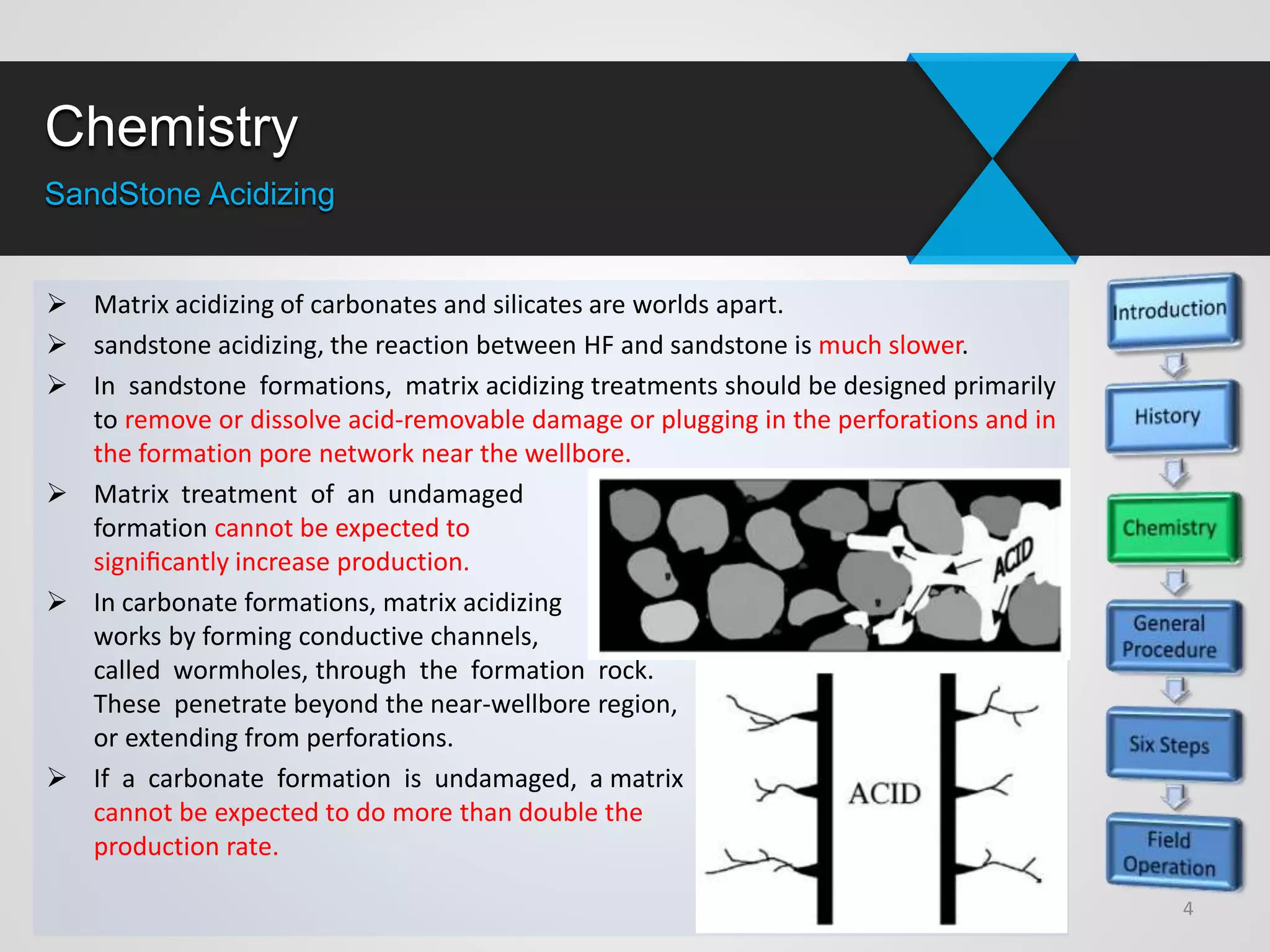
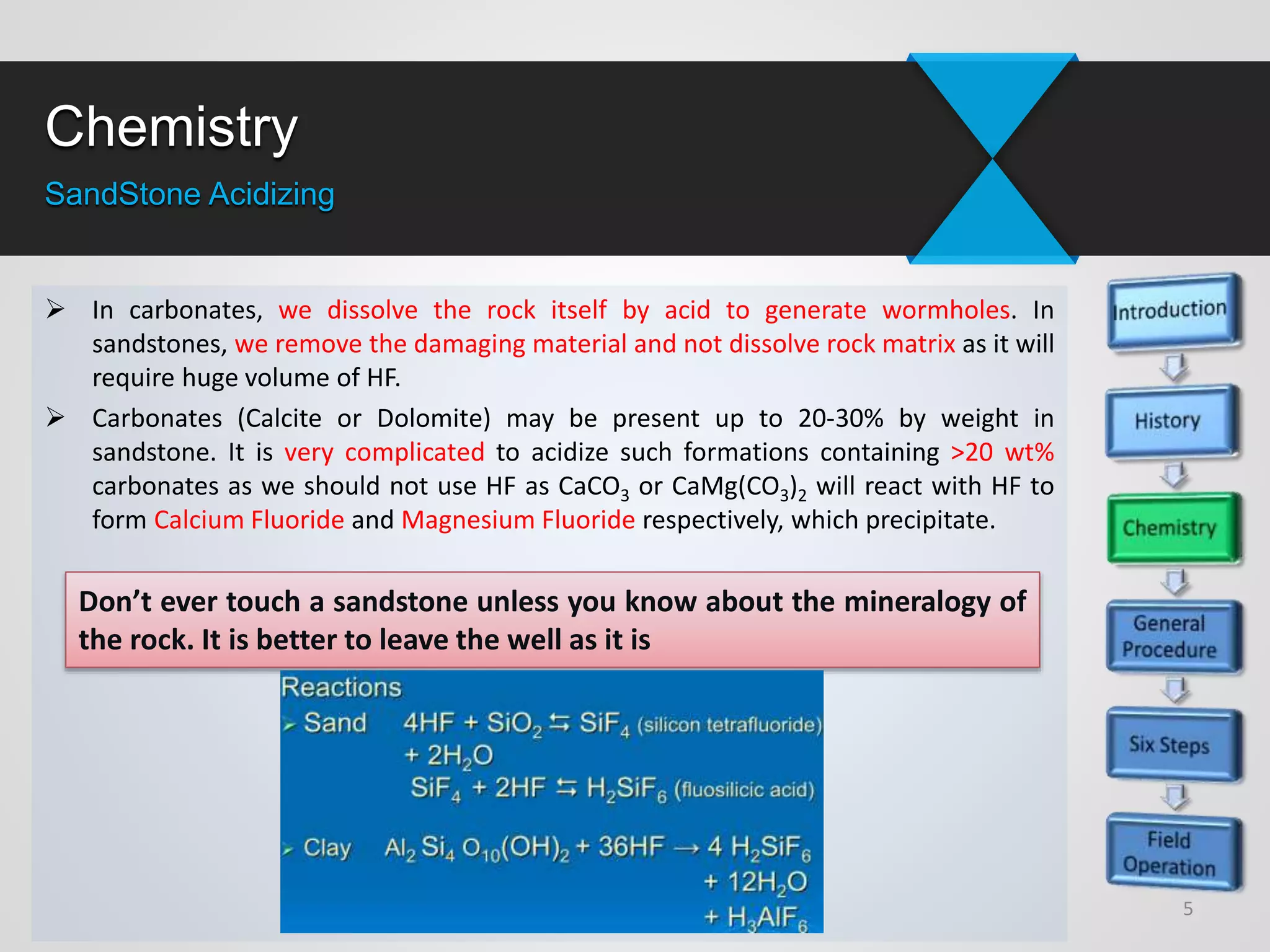
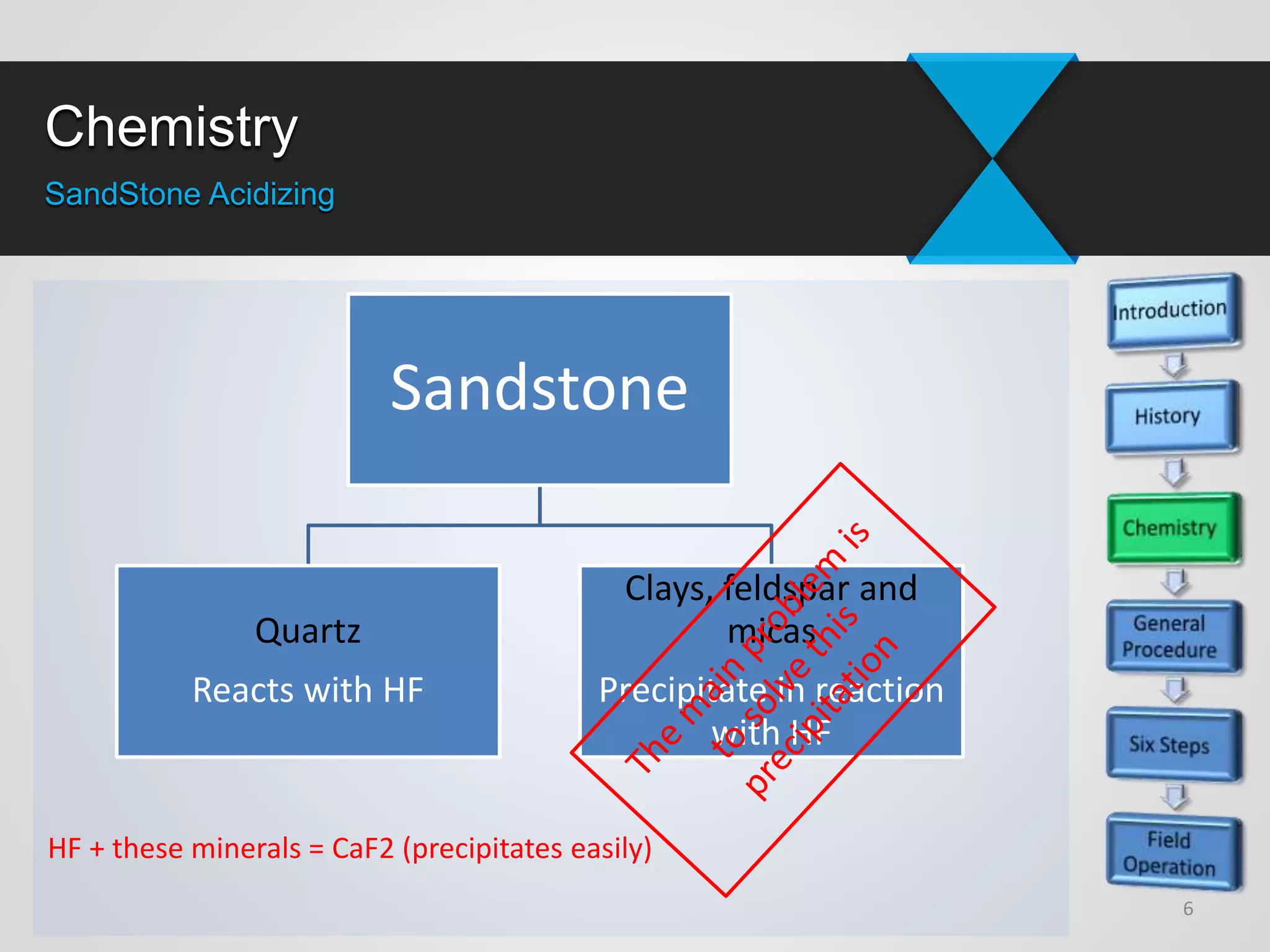
1) Sandstone acidizing involves using acid mixtures to dissolve formation damage and improve permeability in sandstone formations near the wellbore. It aims to remove clay and fine silica particles plugging permeability.
2) The first sandstone acidizing treatment used a mixture of HCl and HF in 1933, but resulted in sand production. Improved practices using tapered HF treatments with HCl preflushes and overflushes were developed in the 1960s.
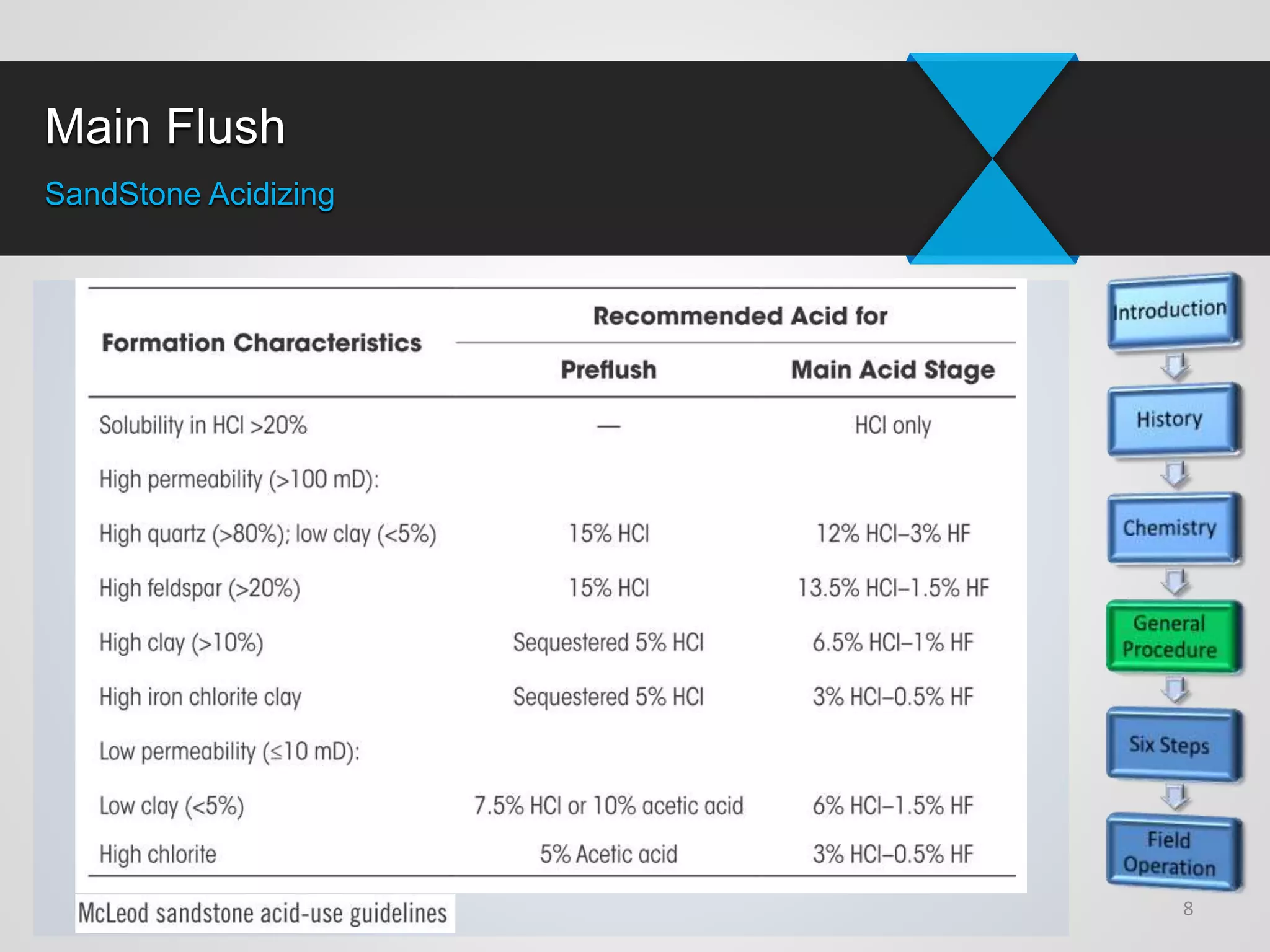
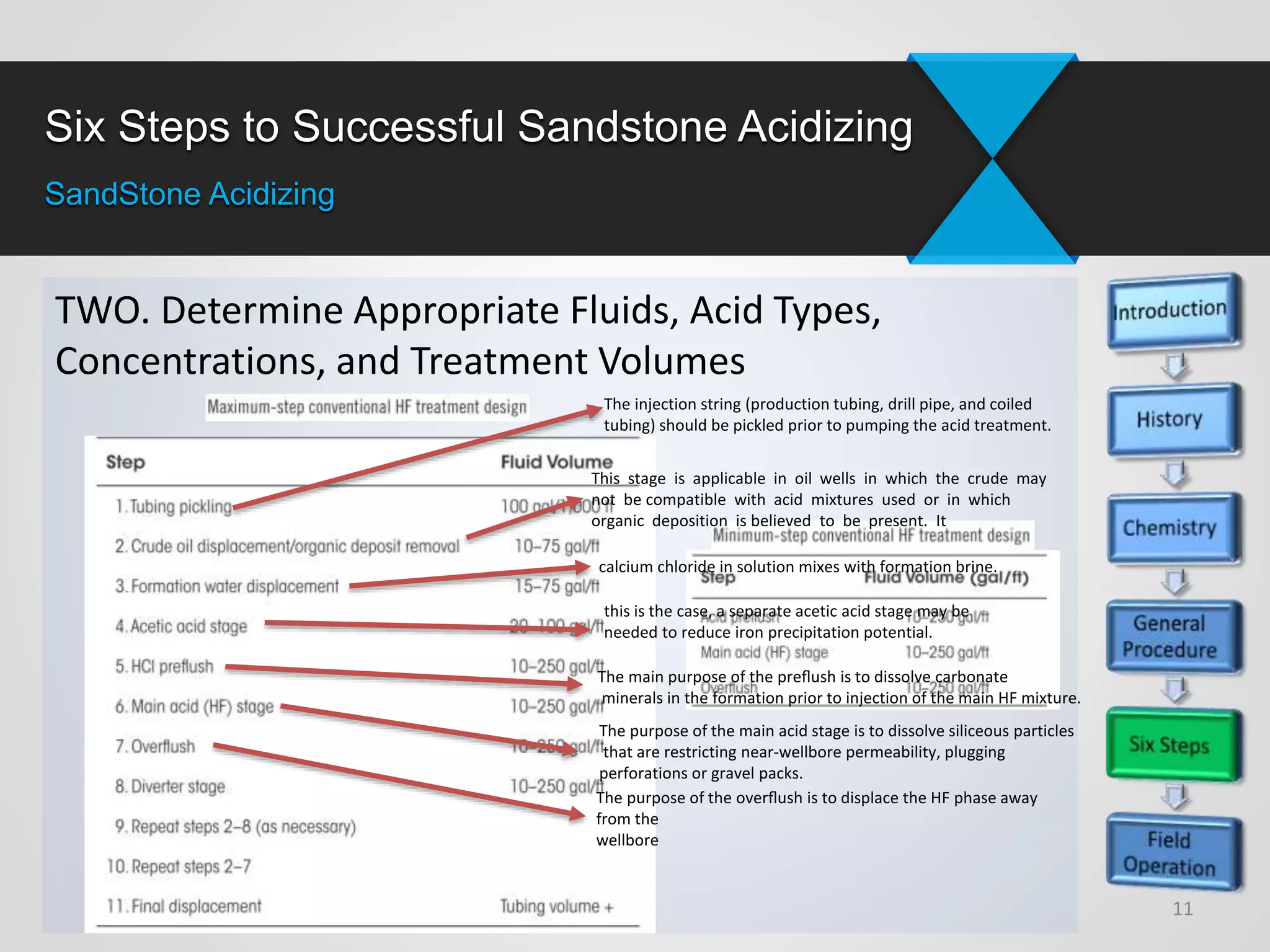
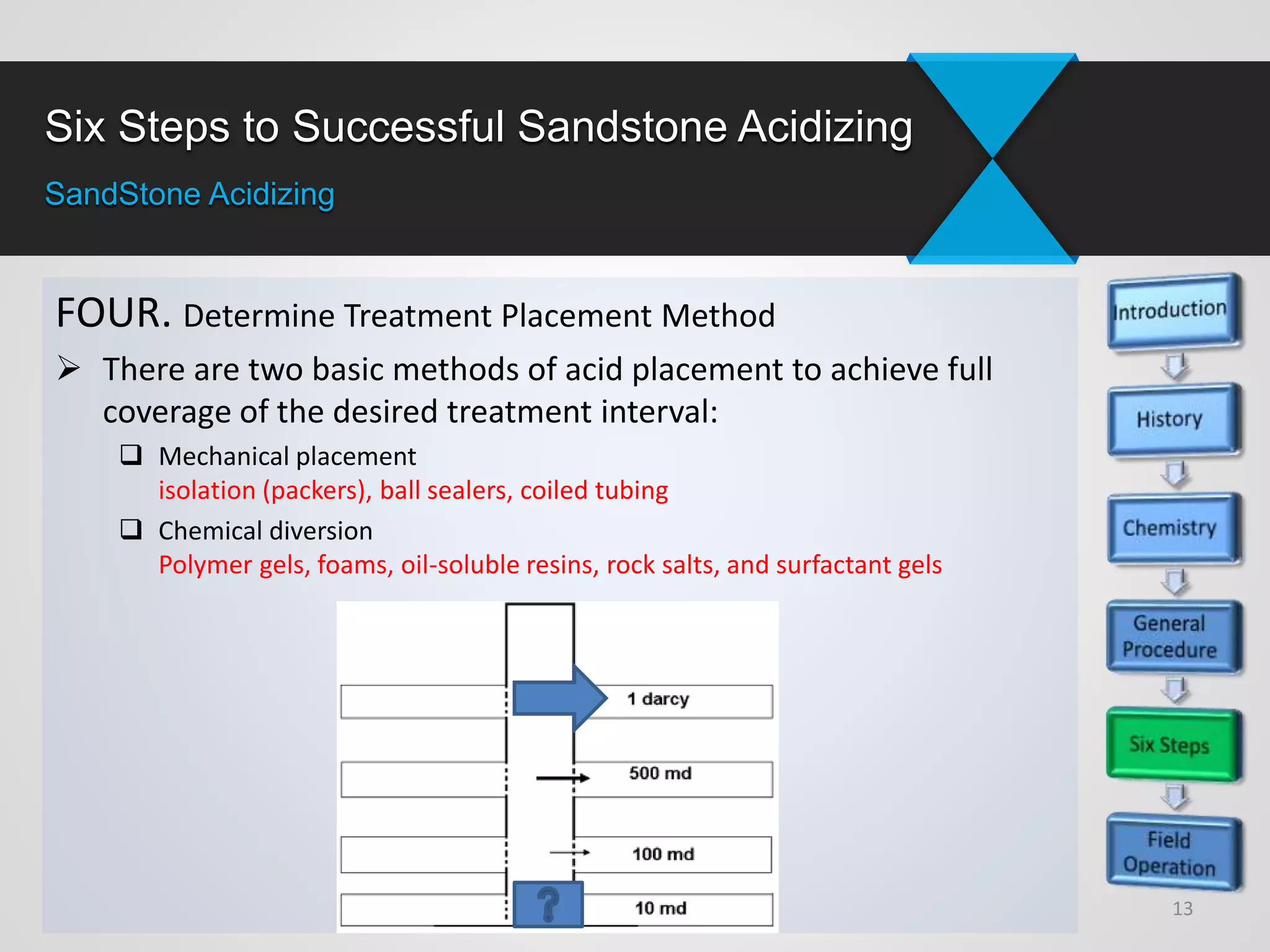
3) Successful sandstone acidizing requires determining whether removable skin is present, selecting the right fluids and volumes, establishing additive programs, properly placing treatments, executing treatments with quality control, and evaluating results.