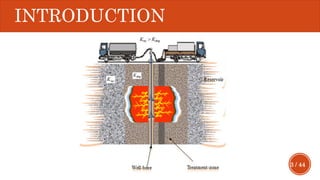
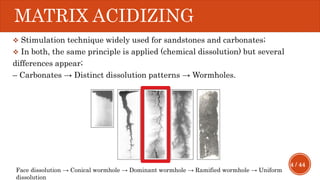
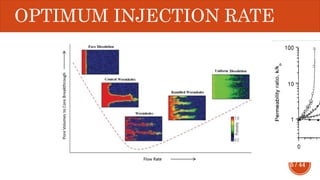
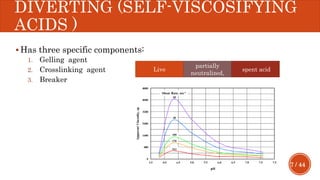
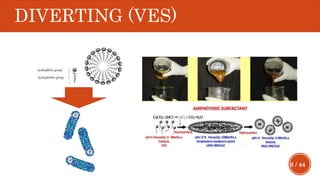
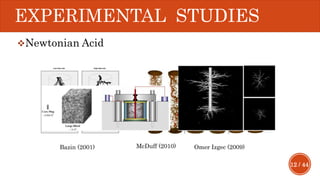
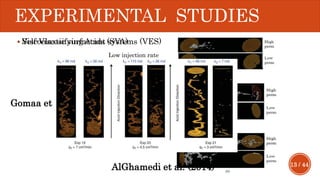
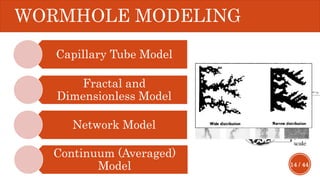
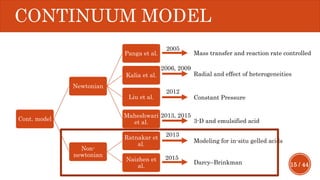
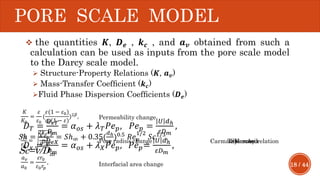
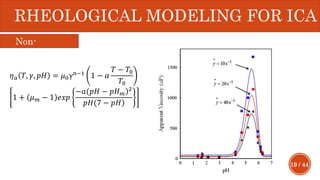
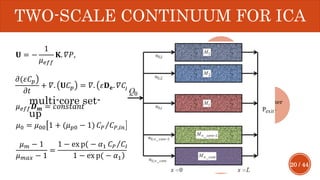
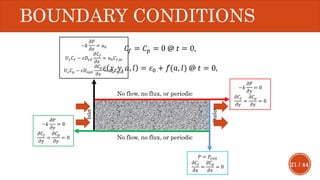
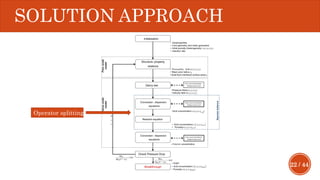
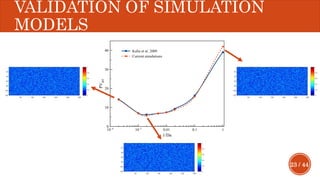
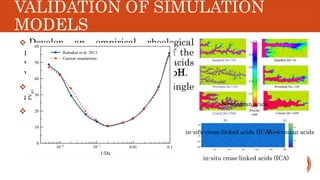
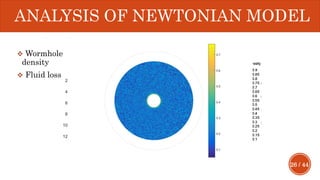
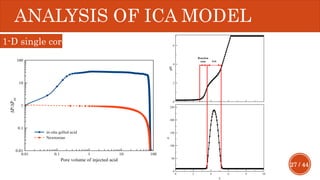
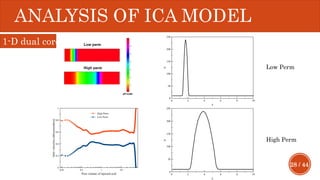
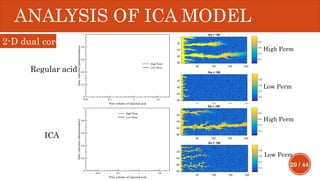
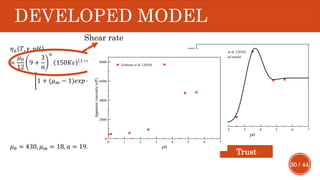
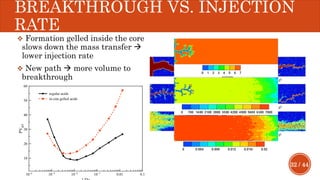
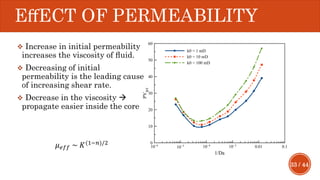
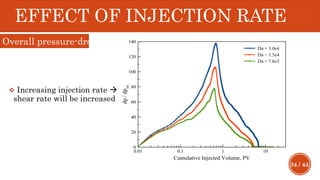
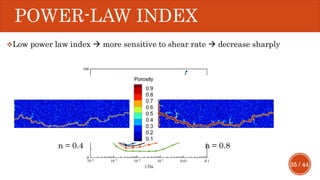
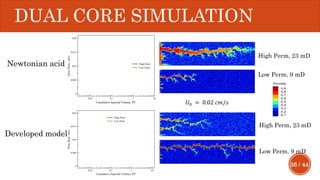
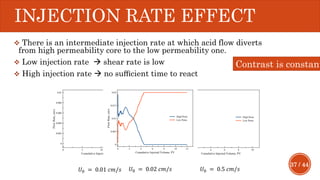
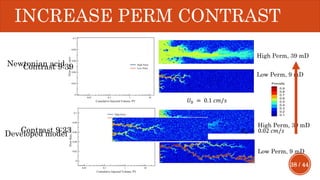
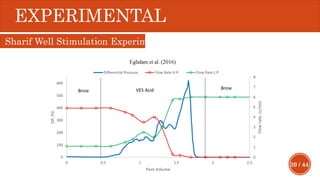
This document presents a developed model for predicting the performance of carbonate matrix acidizing. It begins with an introduction and literature review on experimental and mathematical modeling studies of matrix acidizing. It then describes the development of a continuum model using Darcy's law to model acid transport and reaction in porous media. The model accounts for non-Newtonian rheology of in-situ crosslinked acids. Results are presented on wormhole formation and the effects of injection rate, permeability, and permeability contrast using the developed model. Conclusions drawn include how rheology parameters influence acid diversion. Recommendations include extending the model to include polymer filtration effects.