Embed presentation
Downloaded 341 times



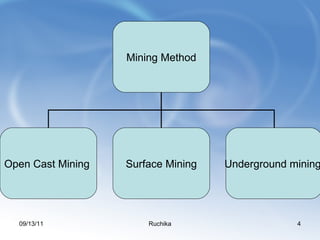


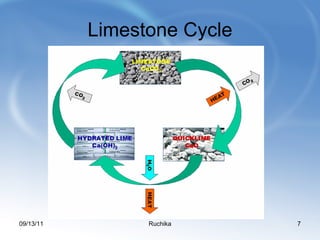

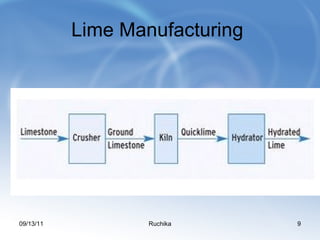

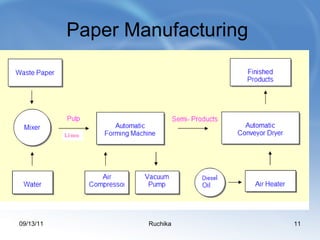
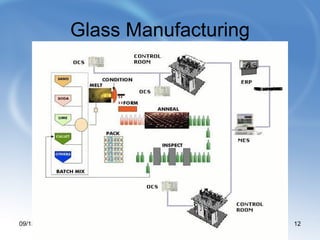

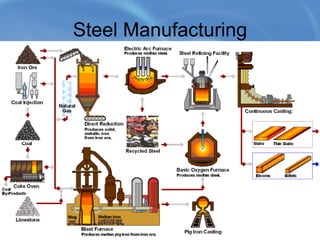
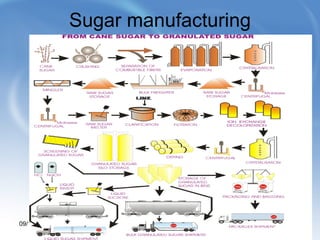



Limestone is a sedimentary rock composed of the shells of dead sea creatures that built up over millions of years. It exists in various forms from soft chalk to hard limestone to the hardest marble. Limestone is mined through both open cast and underground mining. It has many chemical and industrial uses such as in cement, glass, paper, building materials, road construction, and more. Key processes in working with limestone include calcination to produce quicklime, hydration to produce hydrated lime, and reaction with carbon dioxide to produce precipitated calcium carbonate.

















