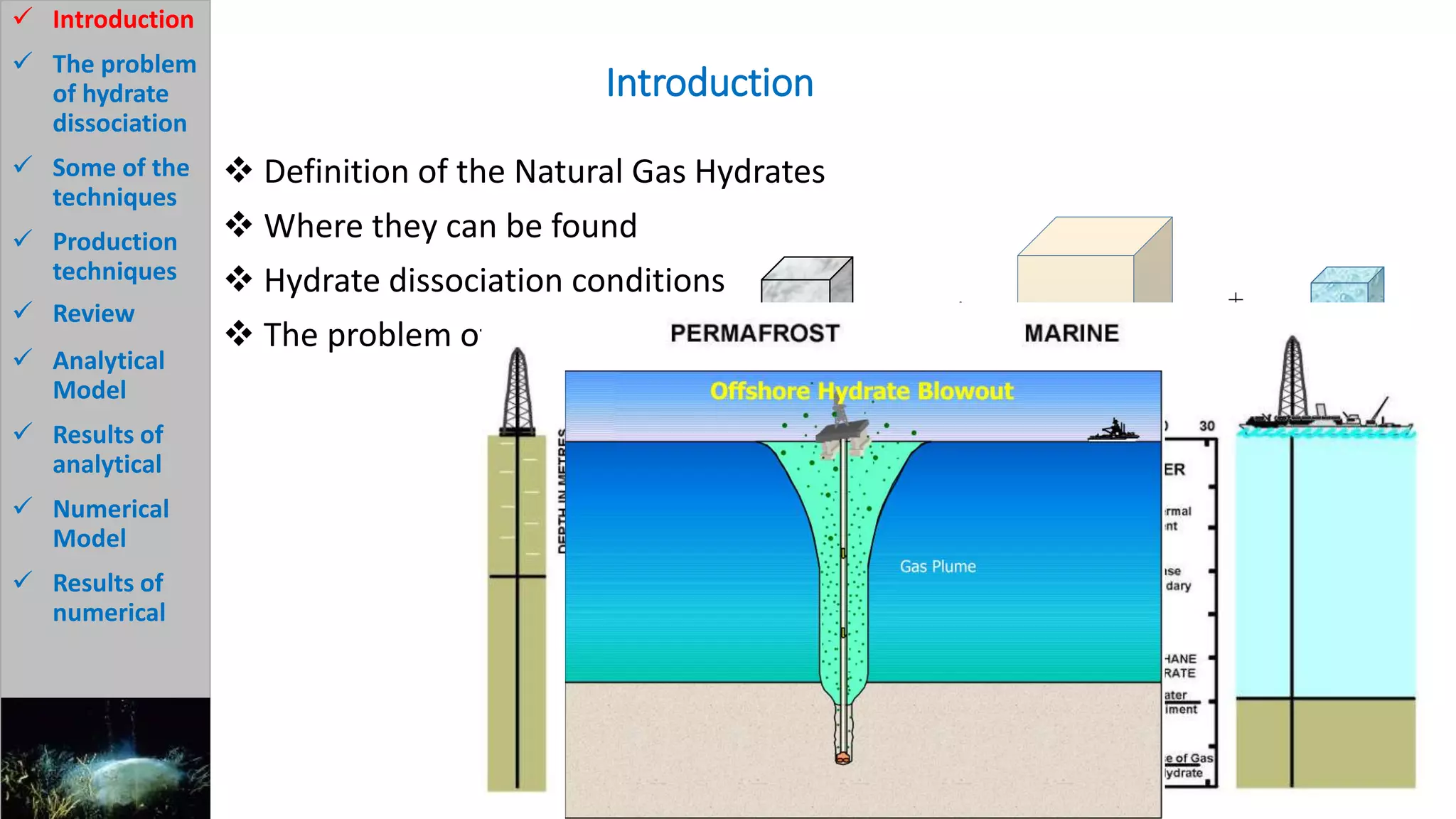
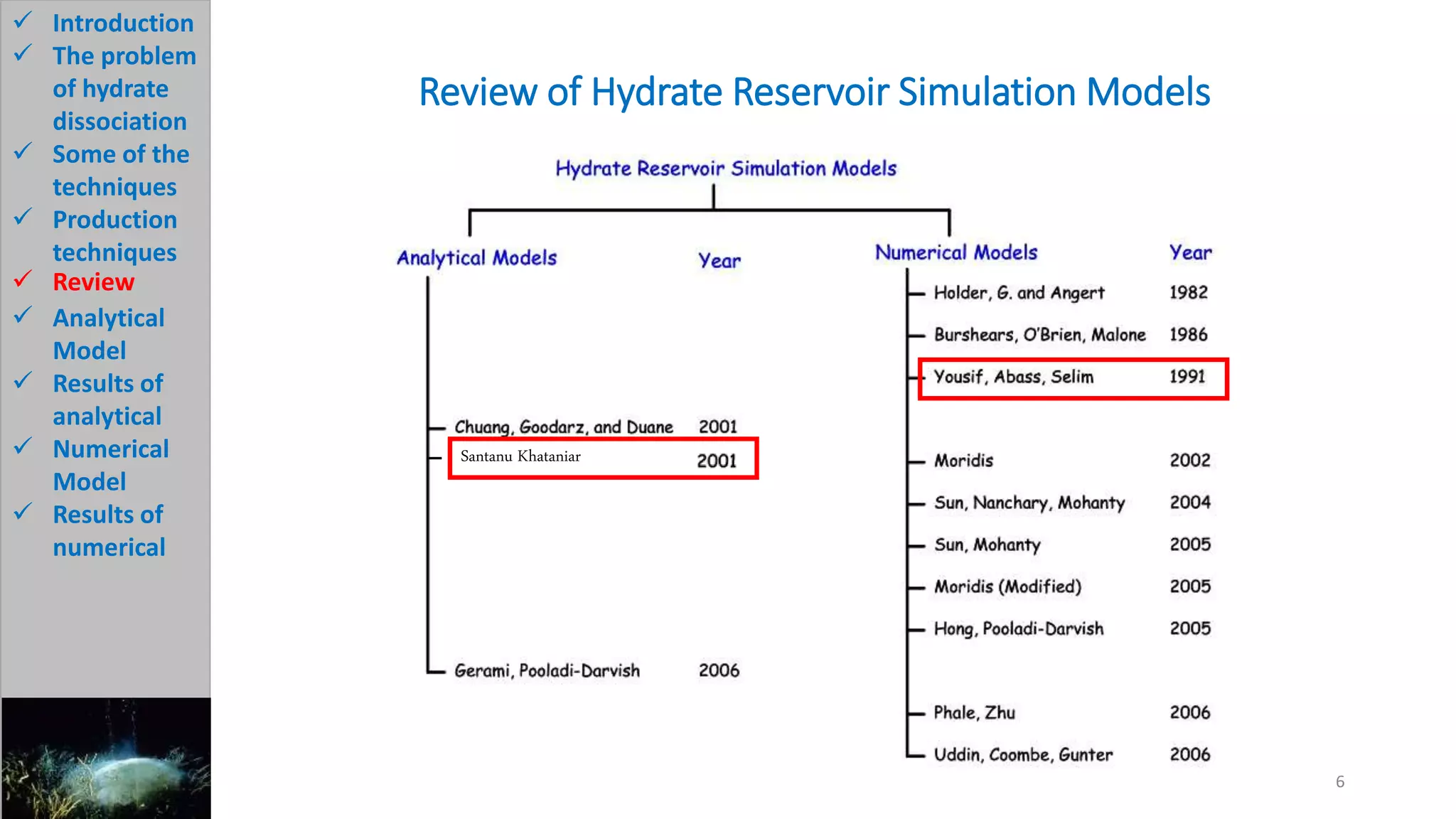
1) The document presents numerical and analytical models for simulating natural gas production from methane hydrate dissociation.
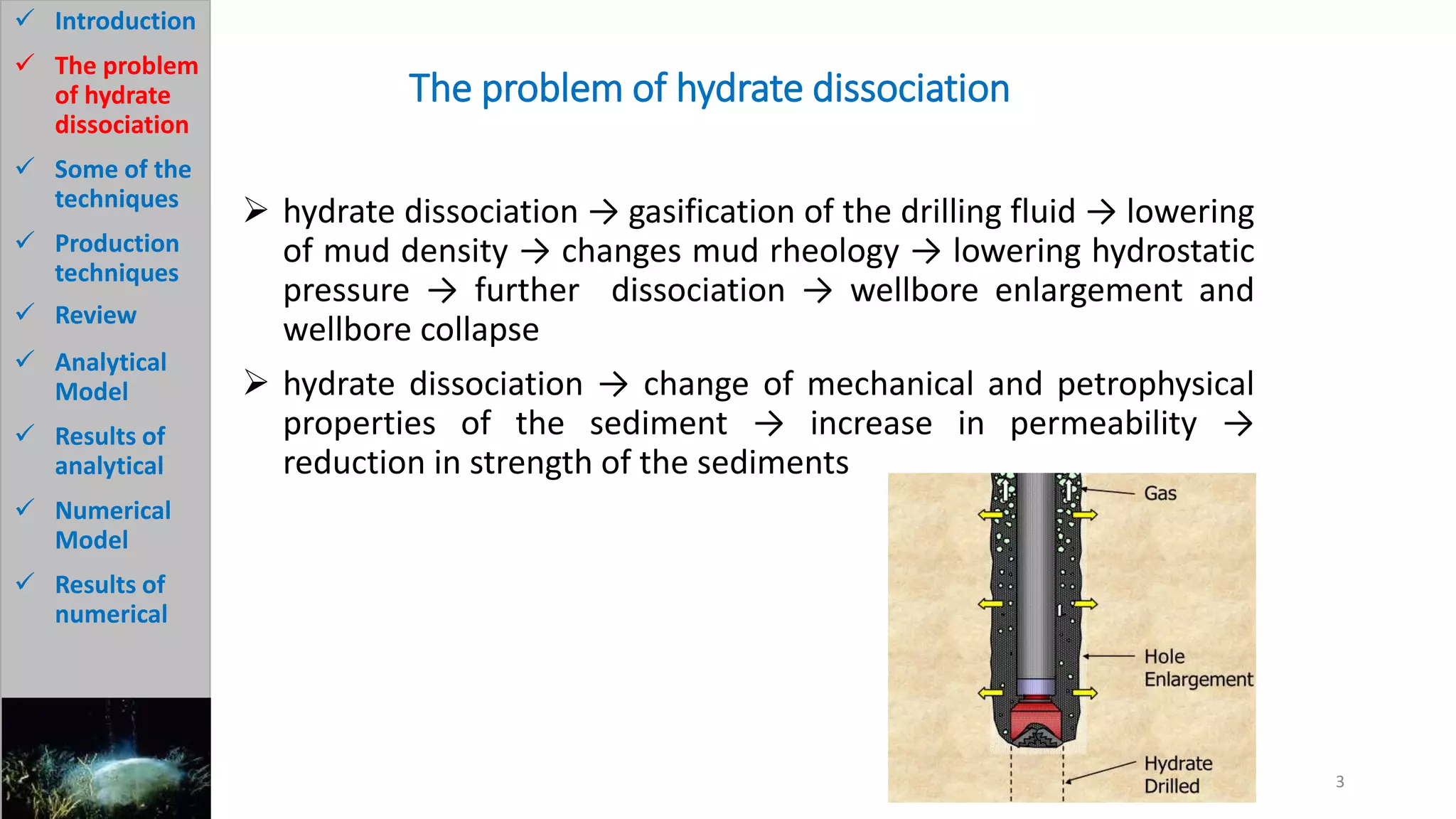
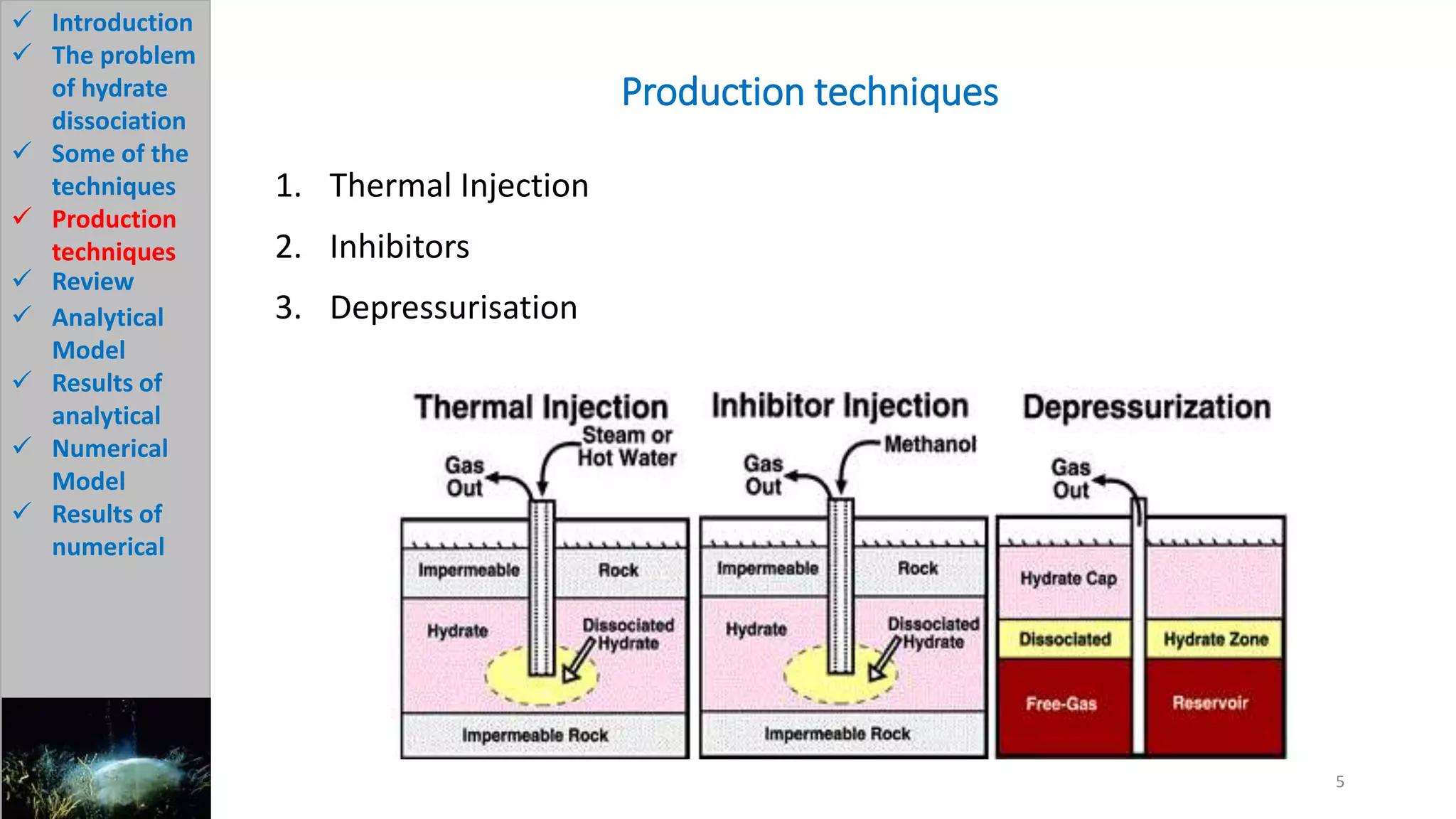
2) It describes the problem of hydrate dissociation in wells, which can cause wellbore collapse if not controlled. Various techniques to avoid this such as cooling drilling fluid or increasing mud weight are also discussed.
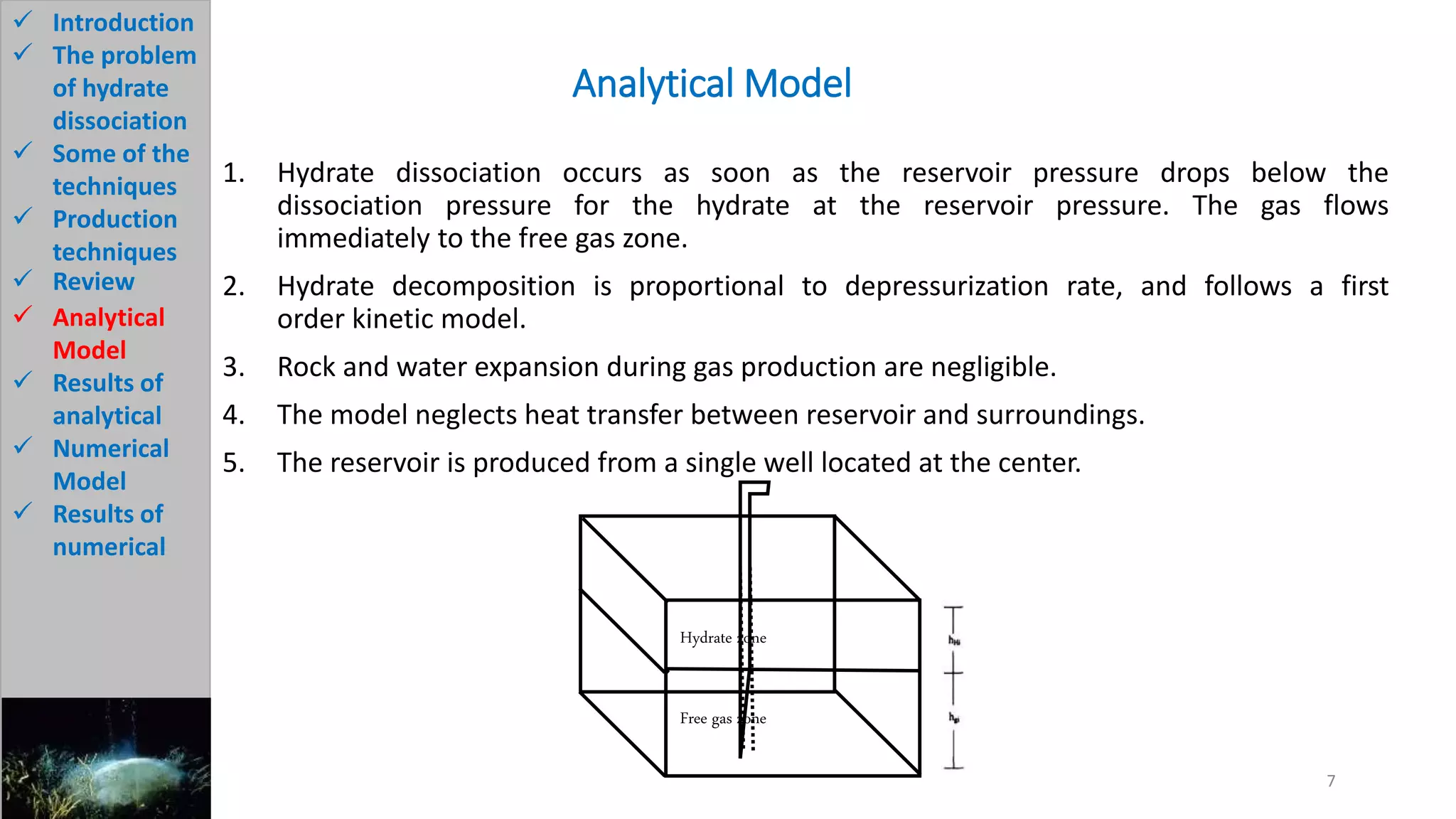
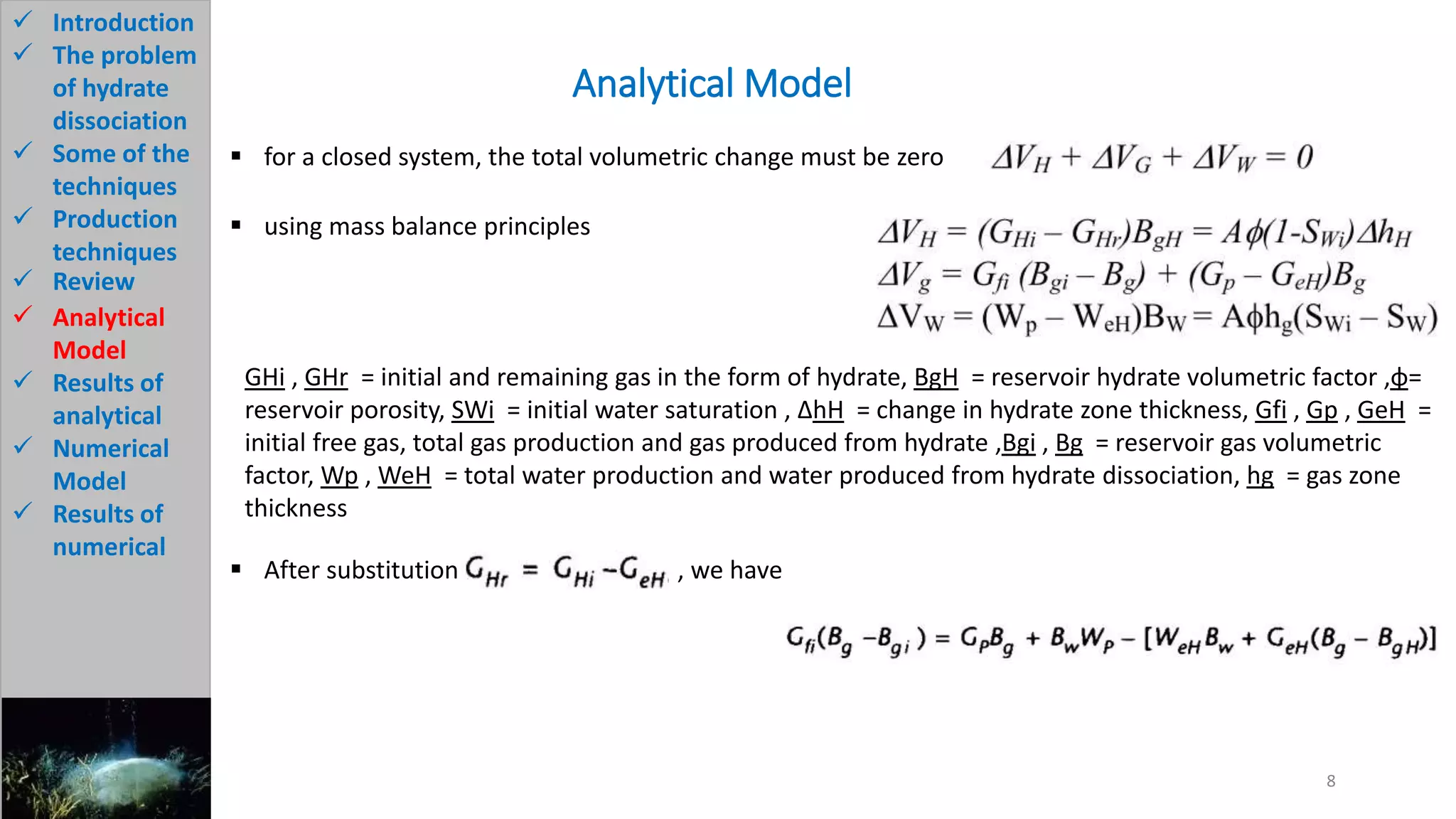
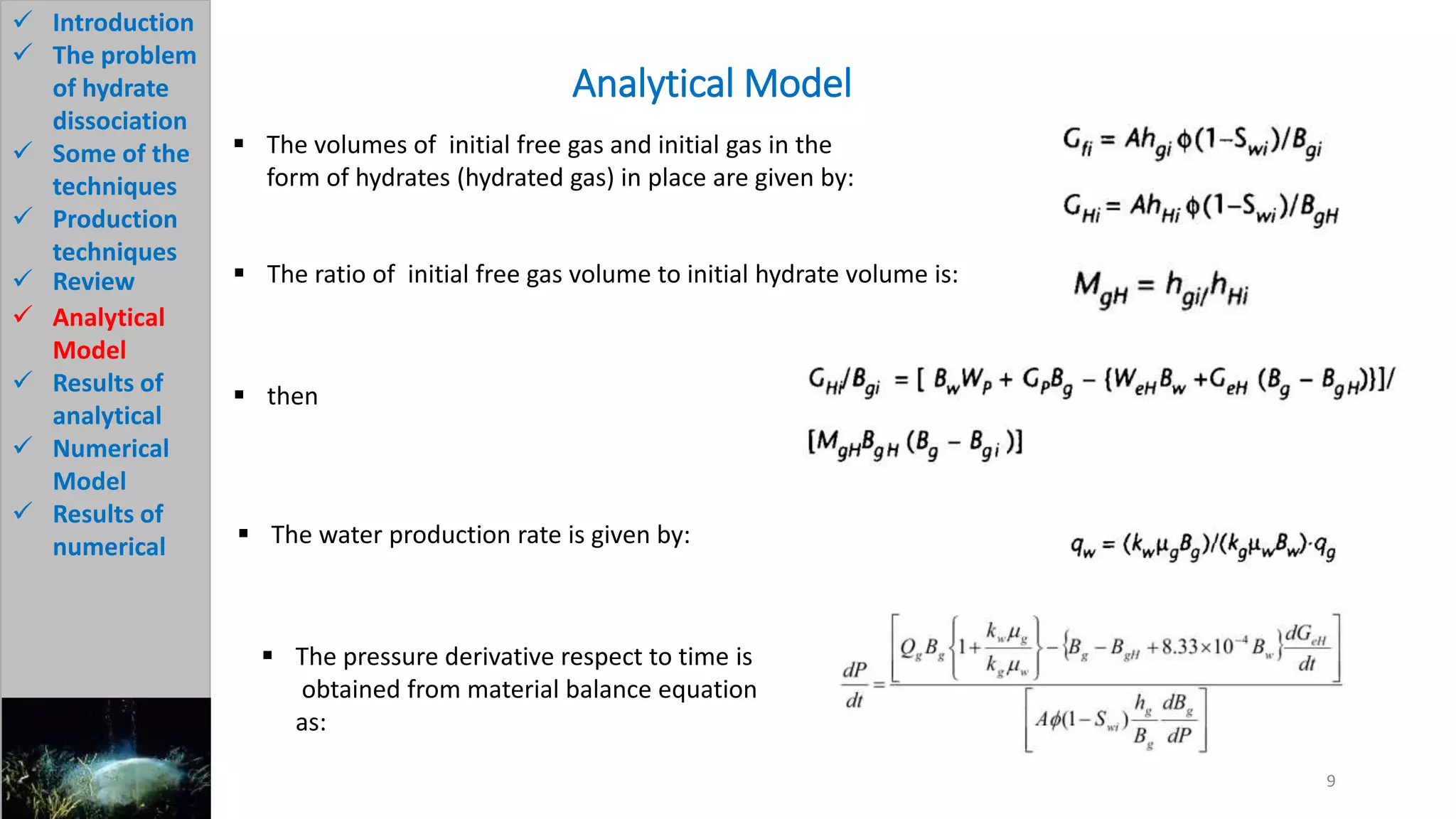
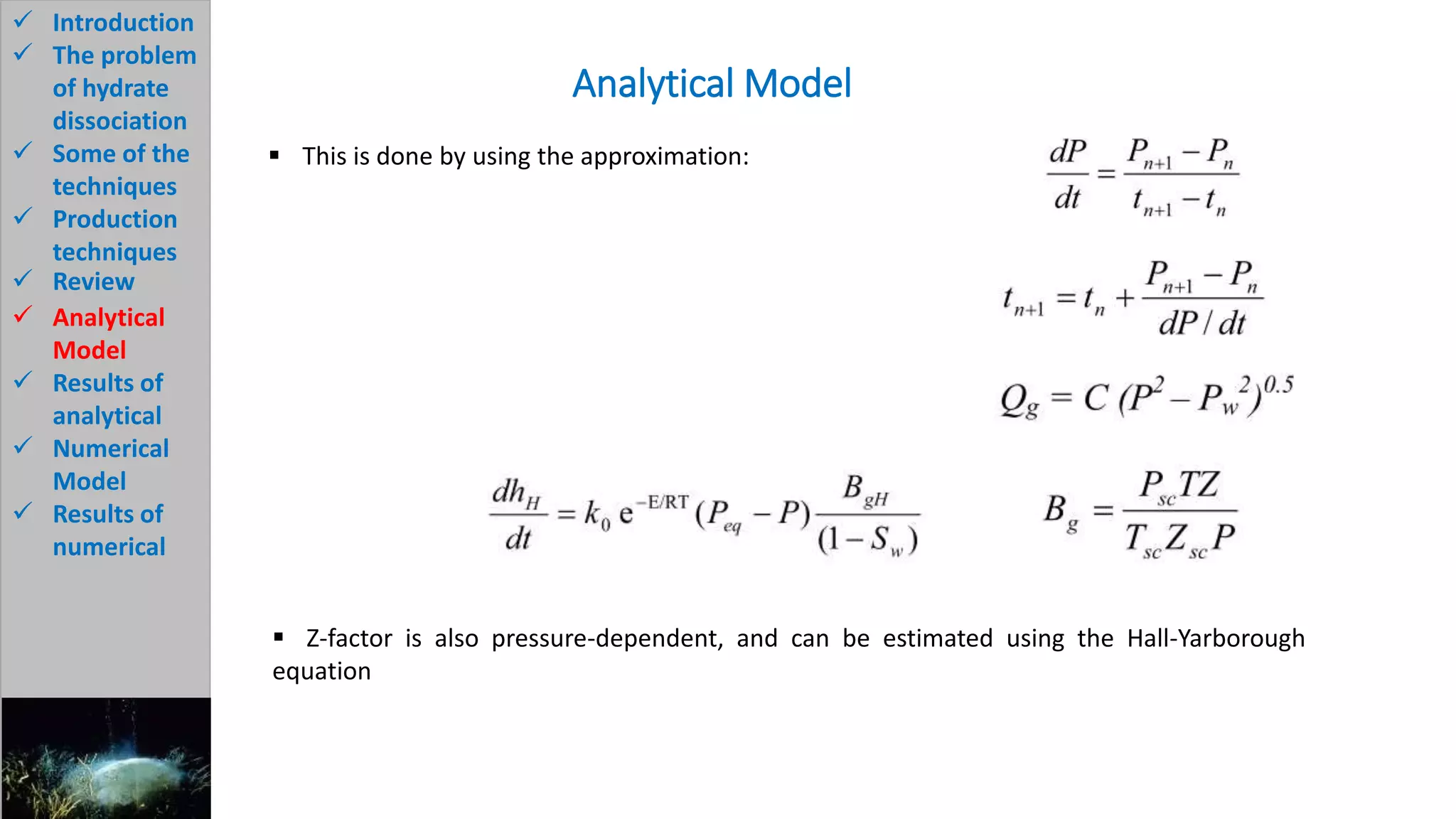
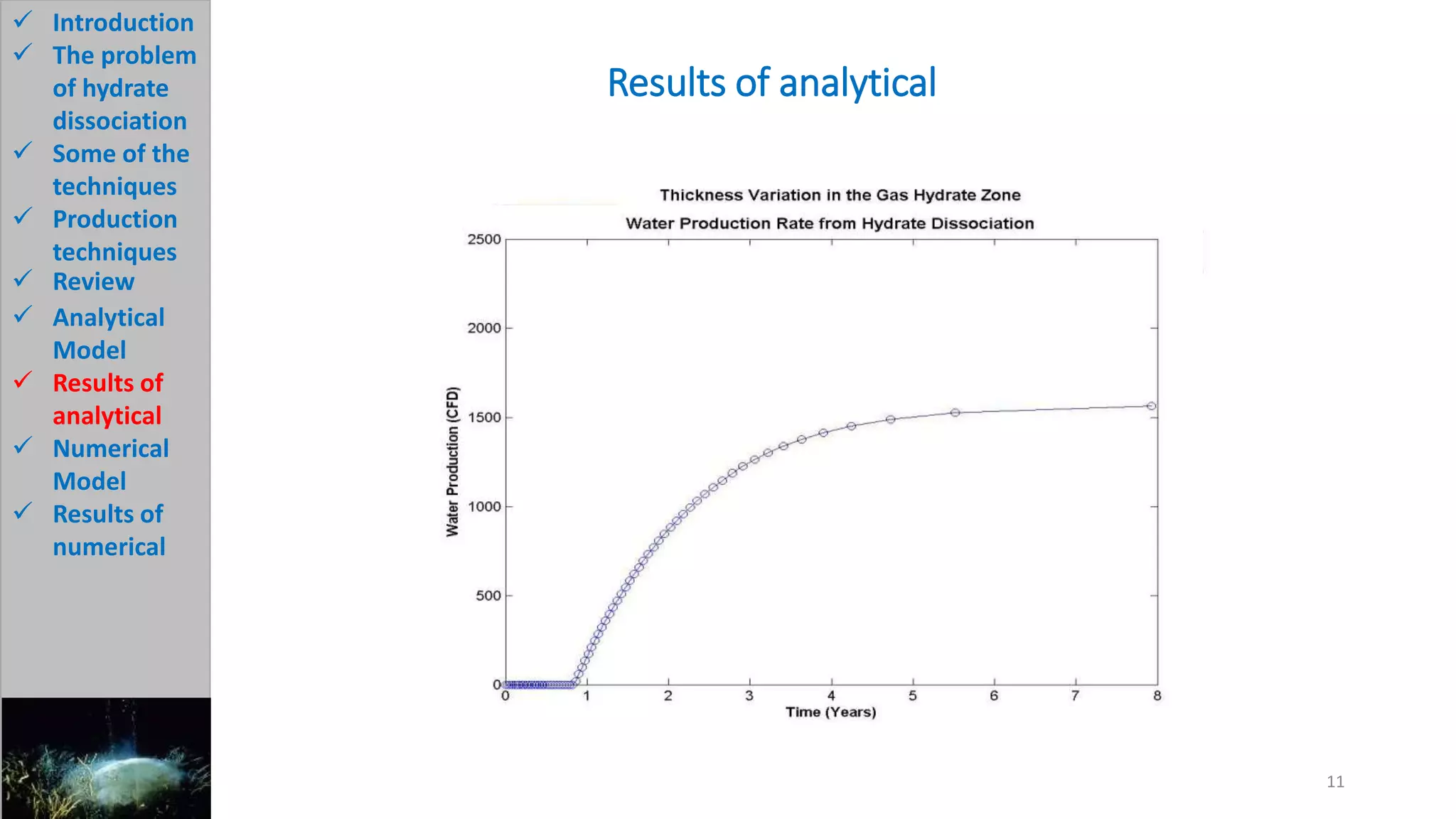
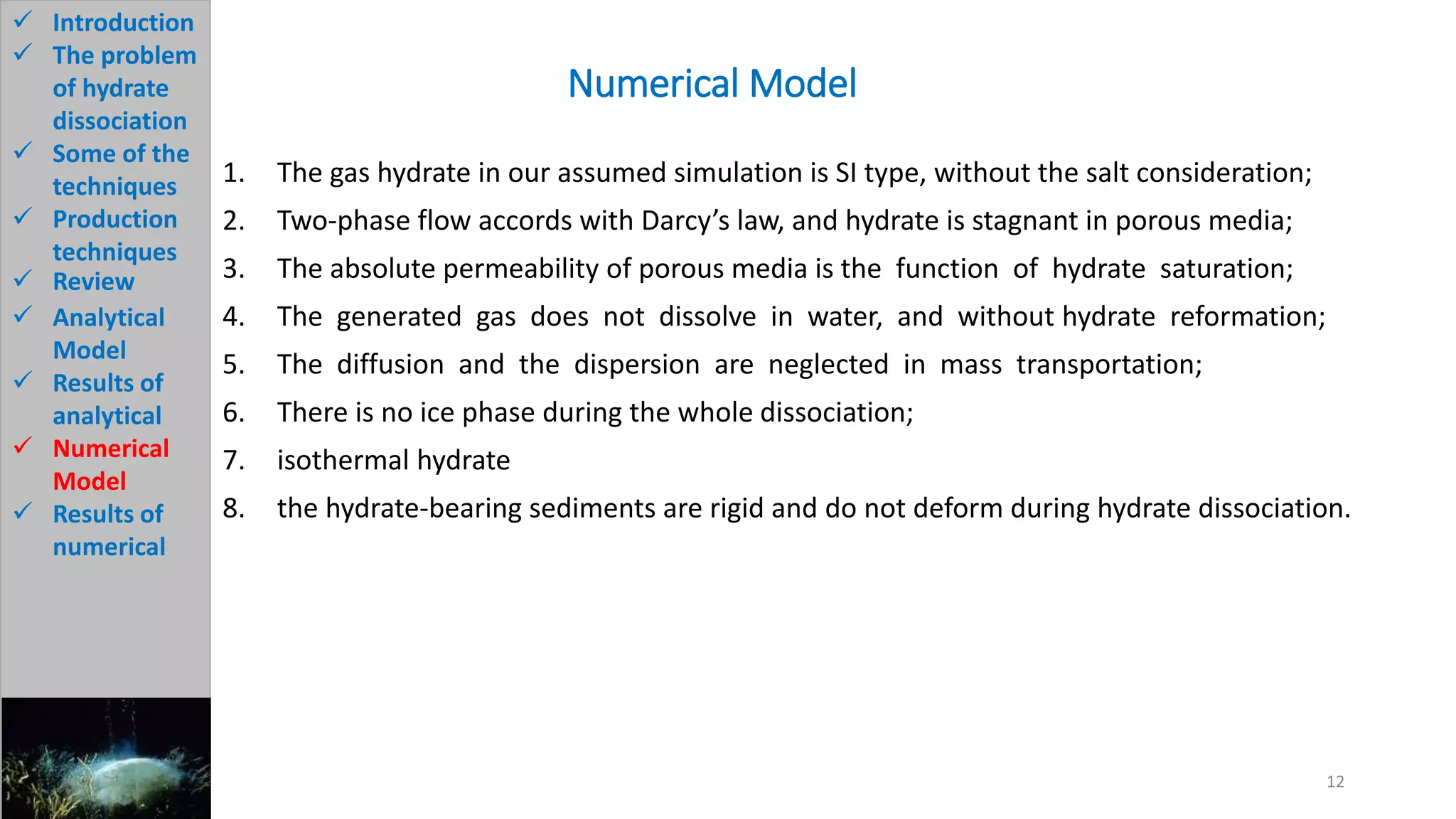
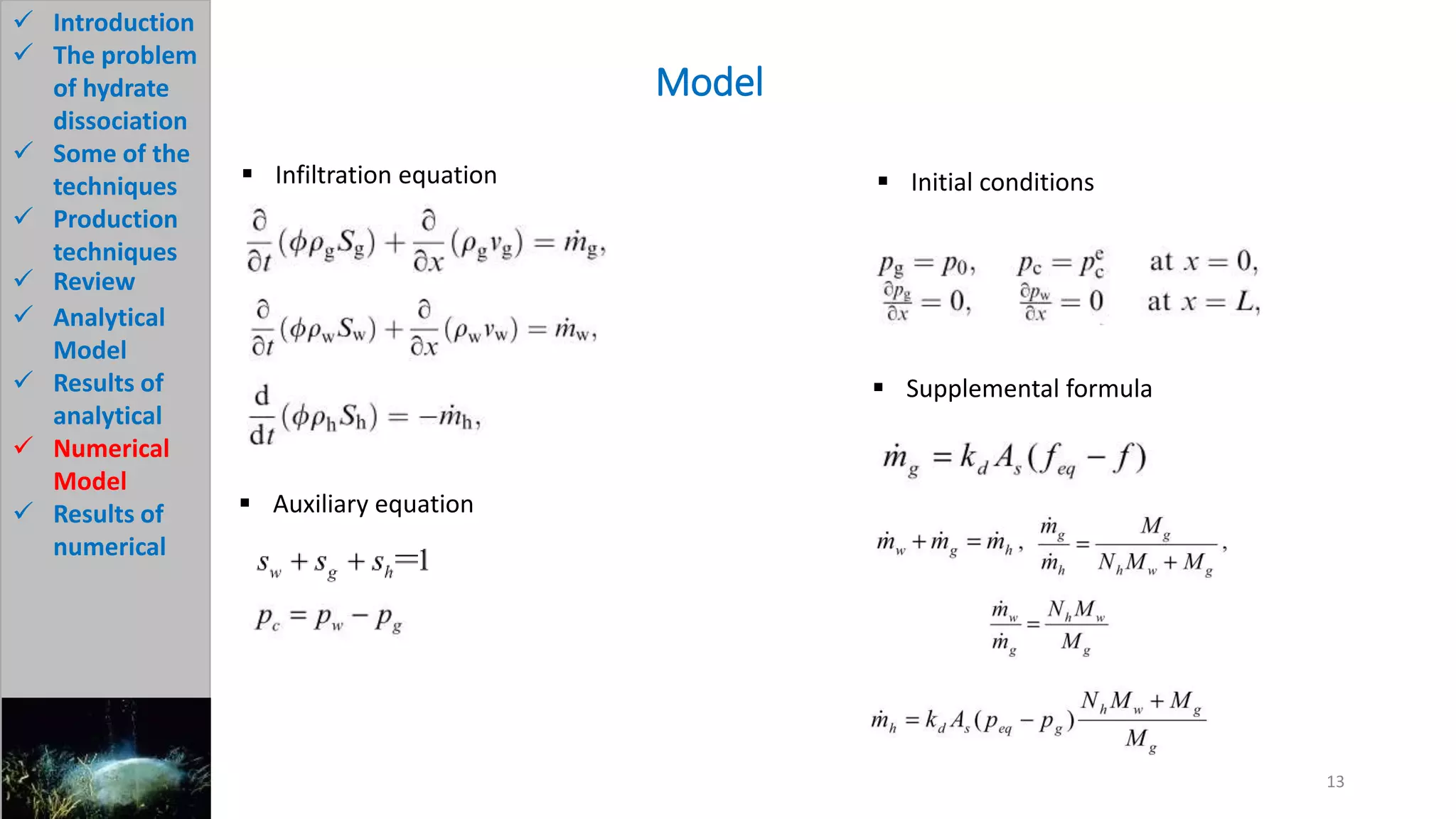
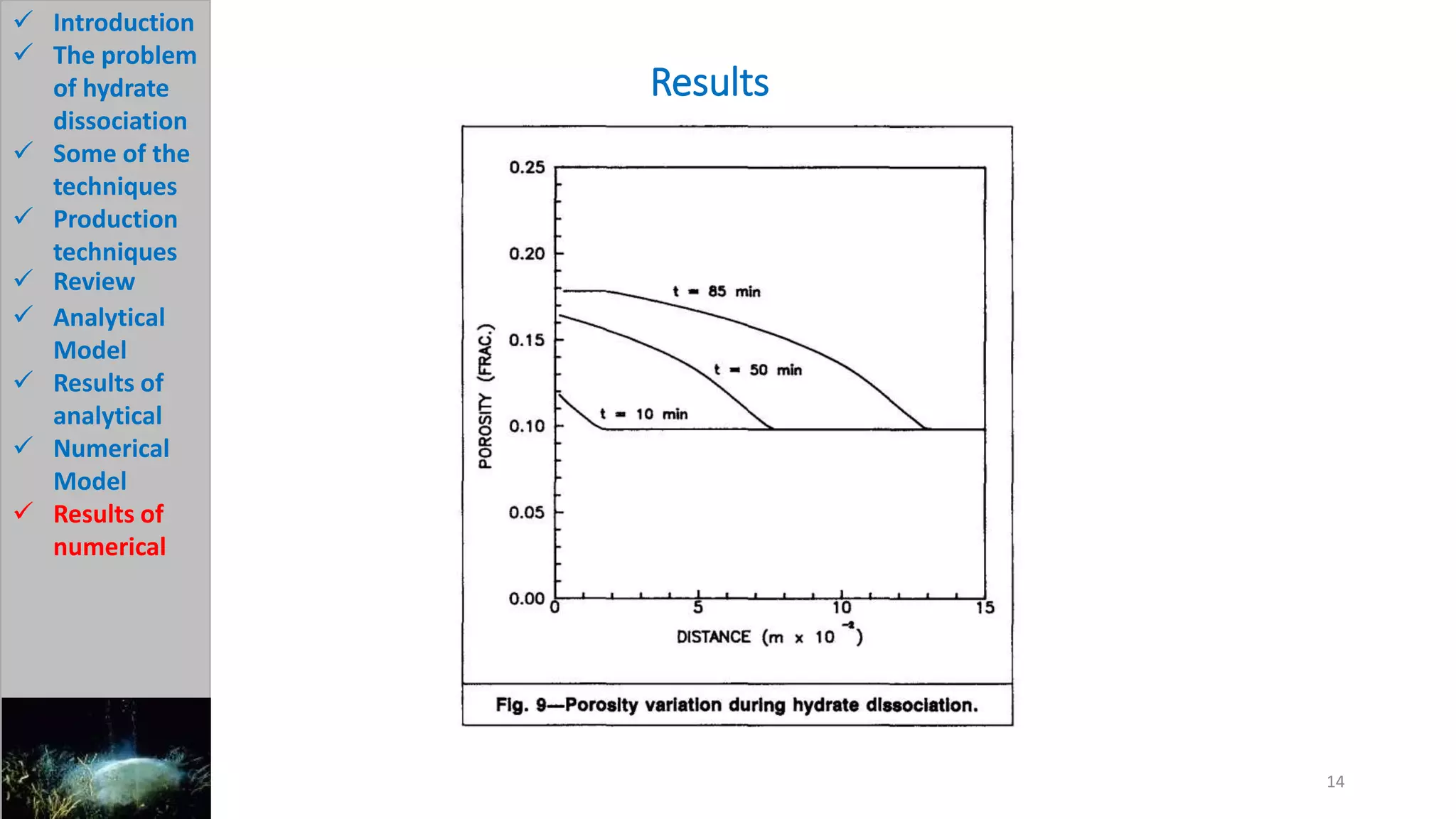
3) The analytical model assumes hydrate dissociation occurs immediately when pressure drops below threshold and follows first-order kinetics. The numerical model sets up governing equations to simulate two-phase flow and permeability changes during dissociation.