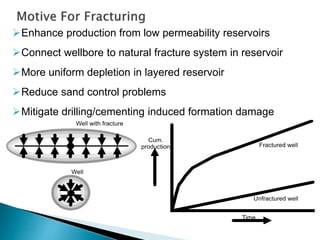
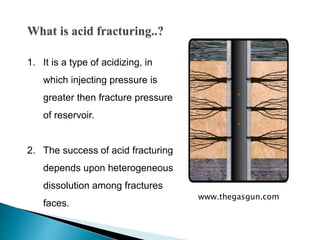
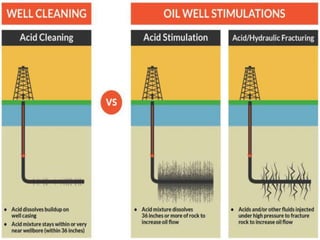
The document discusses acid fracturing, which involves injecting acid at pressures greater than the reservoir's fracture pressure. This connects the wellbore to the natural fracture system and improves production from low permeability reservoirs. Acid fracturing is mostly used in carbonate reservoirs, where fractures are initiated using a fracturing fluid pad followed by acid. Conductivity is achieved through etching of rock minerals on the fracture faces. Factors like fluid leak-off, reaction rate, and temperature affect the etched fracture length. Simulation tools can be used to design treatment schedules to optimize fracture conductivity and length based on reservoir properties and fluid characteristics. Acid fracturing faces challenges like fracture closure and requires proper additive selection. It is widely applied in deep, hard carbon