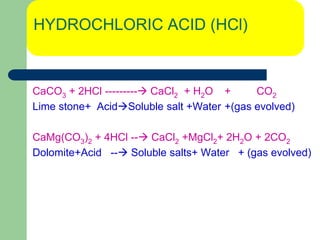
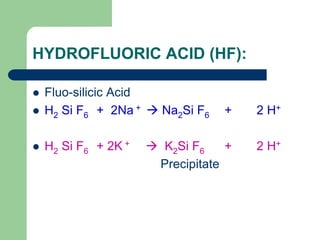
Well stimulation is the process used to enhance the productivity of oil and gas wells through various techniques such as hydraulic fracturing and acidizing. Hydraulic fracturing involves creating conductive paths in low permeability formations to increase oil flow, while acidizing uses acids to remove blockages near well bores and etch fractures for better fluid movement. The document details the methods, objectives, mechanisms, and various additives used in well stimulation processes.












![INITIATION OF FRACTURES
i. Pressure required to create a Horizontal
fracture
Pf (h) = Z = 1.0 x D
Where D is the depth in feet
ii. Pressure required to create a vertical fracture
Pf (v) = [ 2 / (1 - ) ] Z + St
Where is Poisson’s ratio = lateral strain / axial
strain varies between 0.18 to 0.27 for the
average type of rocks.
St = tensile stress.](https://image.slidesharecdn.com/well-stimulation-240426222128-780aaf1b/85/Well-Stimulation-Well-Stimulation-Well-Stimulation-13-320.jpg)