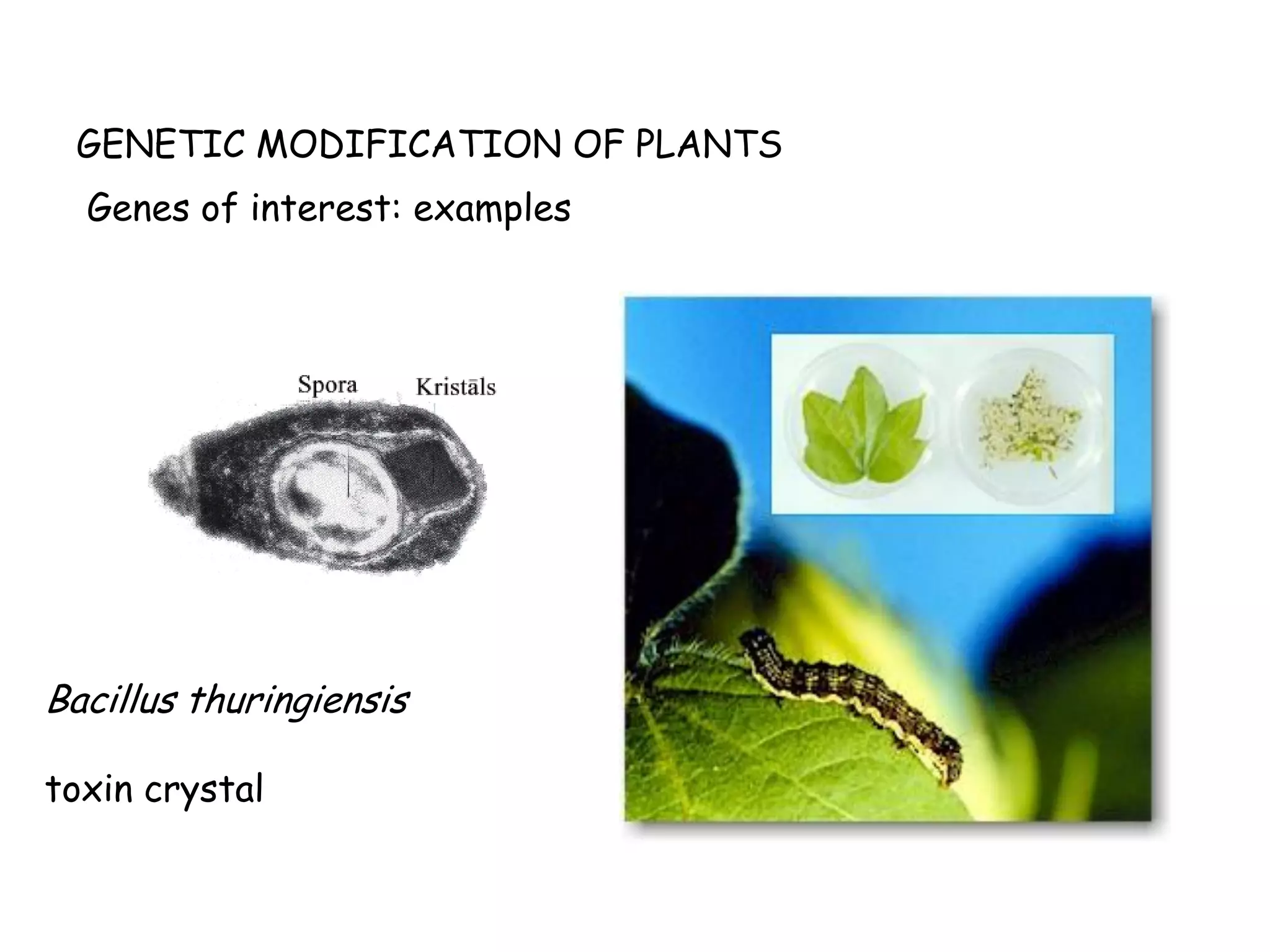
This document discusses genetically modified organisms (GMOs) in food and agriculture. It provides background on GMOs, including sources of GM food like microorganisms, animals, and plants that have been genetically engineered. It then discusses the genetic modification process and genes commonly used. The document outlines potential risks of GMOs, including health, environmental, socioeconomic, and ethical issues. It also discusses the precautionary principle for assessing GMO risks and regulations around GMOs in the European Union.