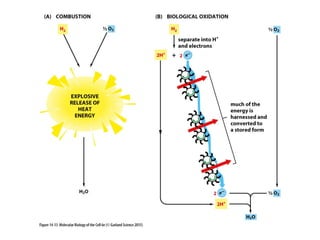
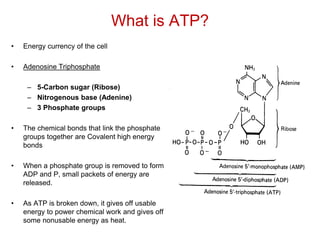
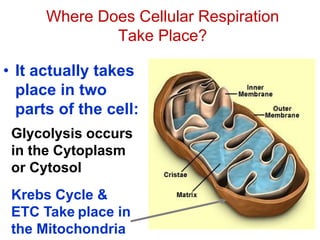
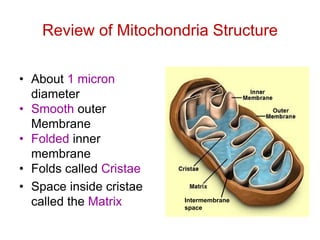
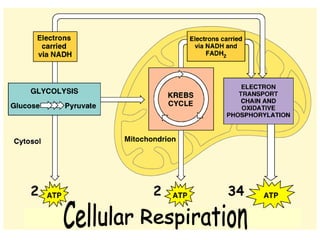
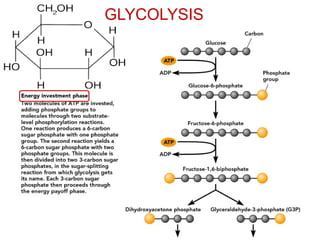
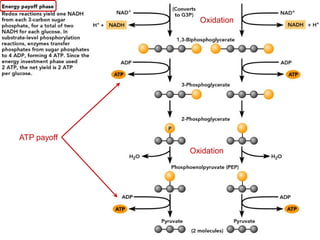
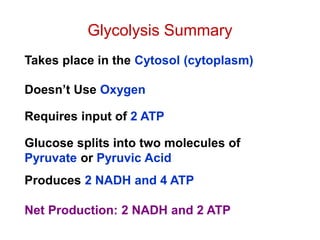
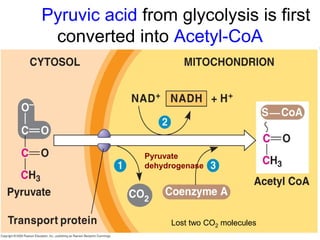
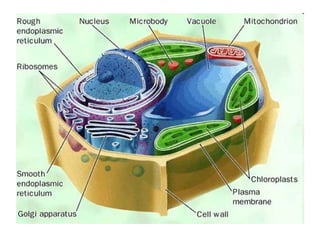
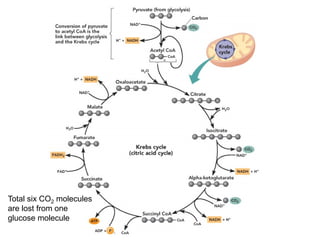
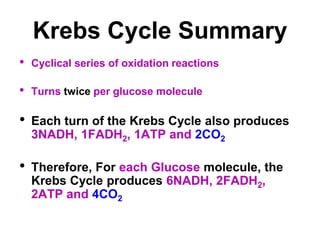
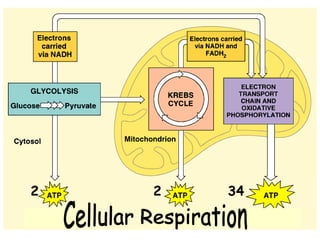
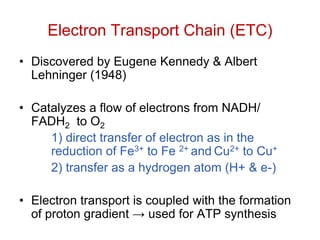
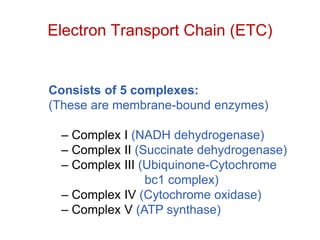
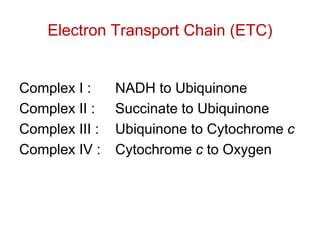
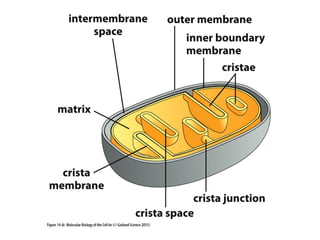
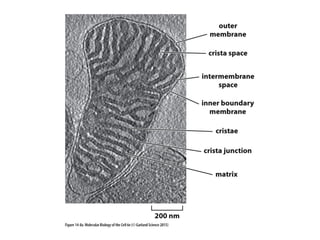
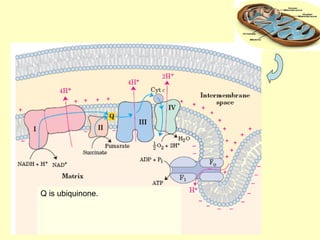
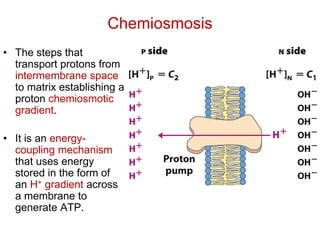
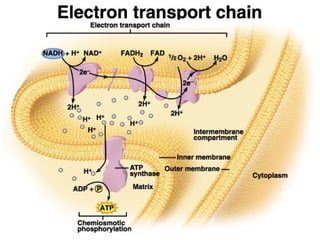
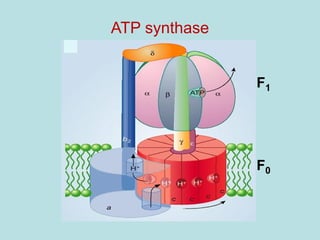
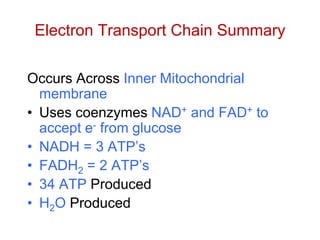
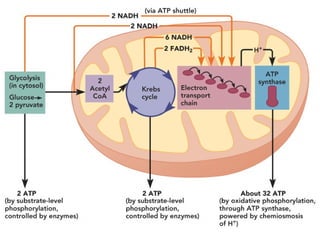
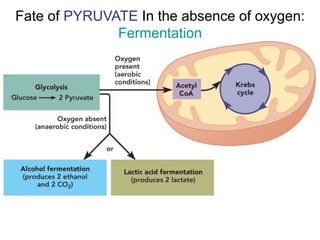
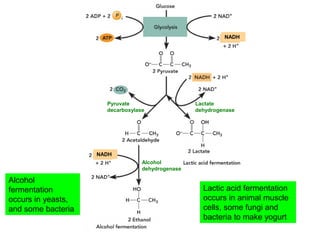
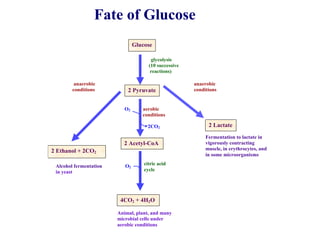
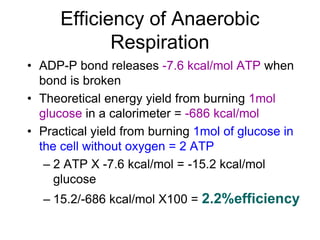
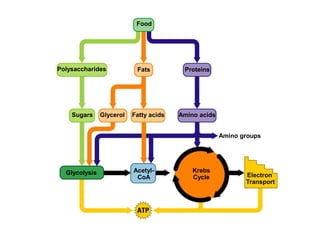
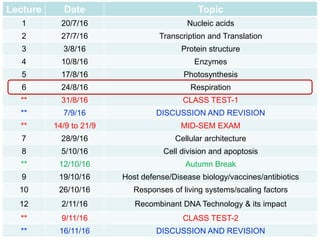
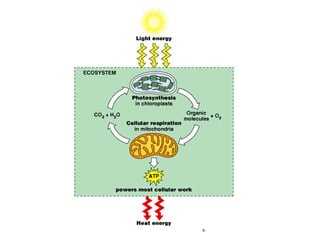
This document contains the schedule and topics for a course on the Science of Living Systems. The course covers topics such as nucleic acids, transcription and translation, protein structure, enzymes, photosynthesis, respiration, cellular architecture, cell division, and recombinant DNA technology. Key concepts around respiration are summarized, including that it converts food energy into ATP through glycolysis, the Krebs cycle, and the electron transport chain in the mitochondria. Fermentation is discussed as an anaerobic pathway that yields less ATP than aerobic respiration. The efficiencies of aerobic and anaerobic respiration are compared. Cellular respiration can break down various biomolecules, and its commercial uses include producing wine, beer and bread.


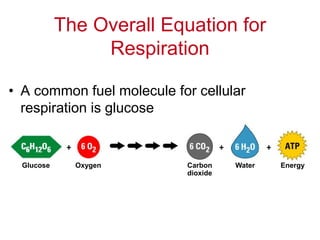
![[Oxygen gains electrons (and hydrogens)]
Oxidation
[Glucose loses electrons (and hydrogens)]
Glucose Oxygen Carbon
dioxide
Water
Reduction](https://image.slidesharecdn.com/lecture6respirationemailed-170211181606/85/Respiration-7-320.jpg)