Embed presentation

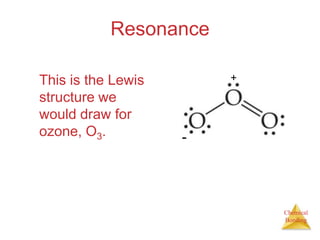
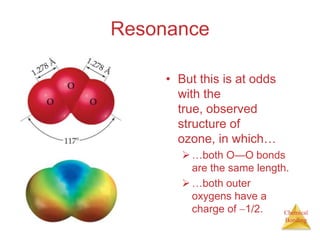
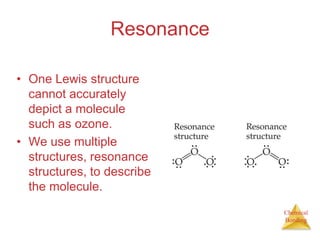
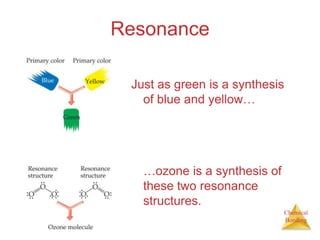
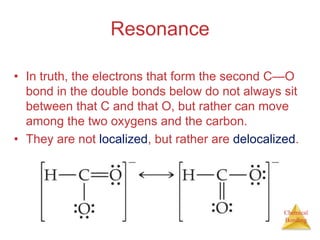
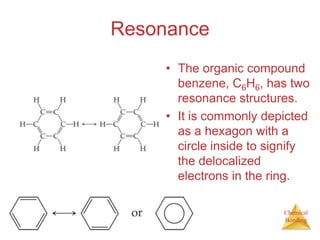
This document discusses the concept of resonance in organic chemistry. Resonance describes when the bonding in a molecule cannot be accurately represented by a single Lewis structure, and instead requires multiple structures that depict the delocalized electrons. Ozone is provided as an example where two resonance structures are needed to represent its true structure with equal bond lengths and partial charges on the outer oxygens. Benzene is also described as having two resonance structures, and its hexagonal structure with a circle inside represents the delocalized pi electrons in the ring.






