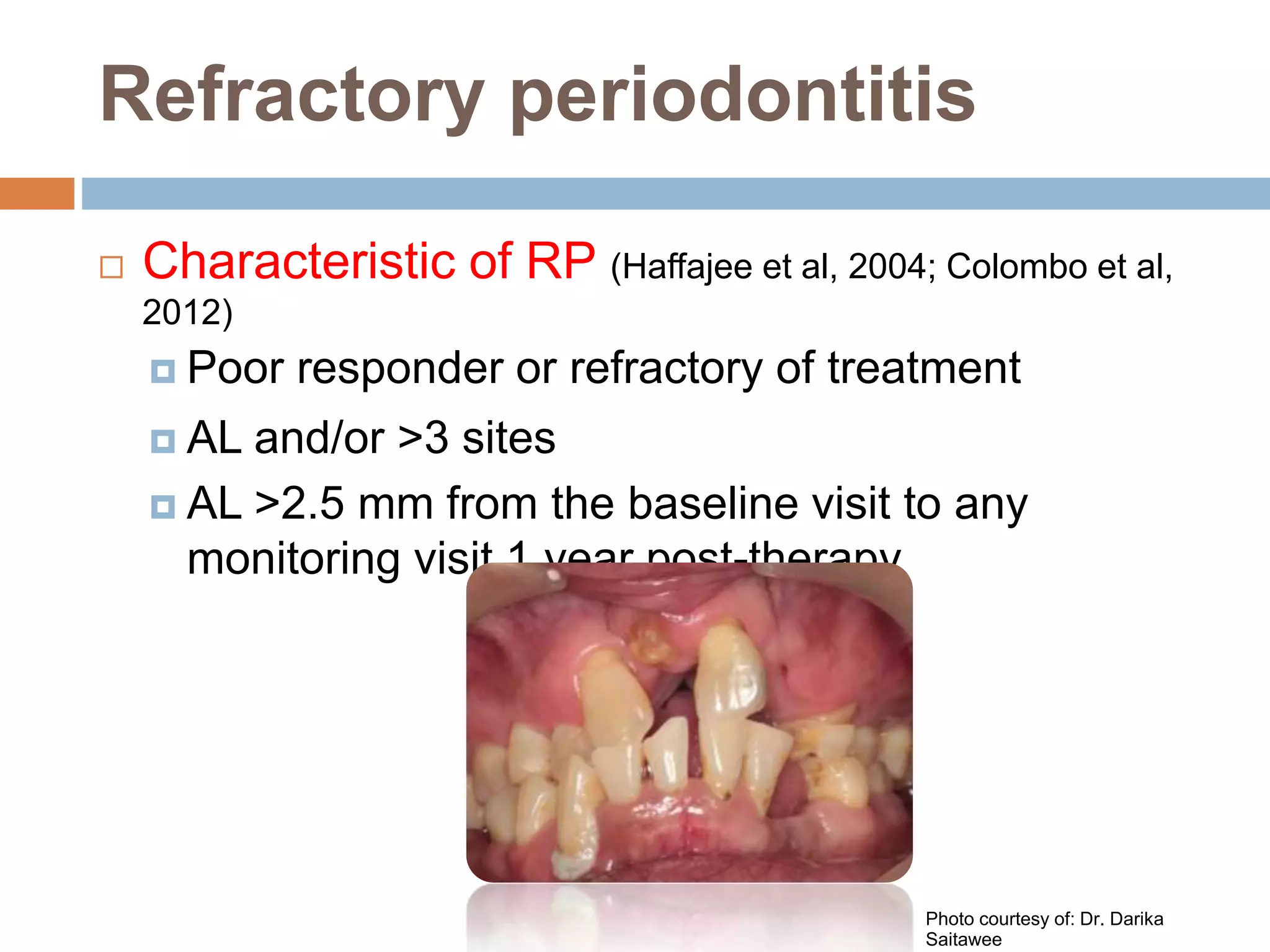
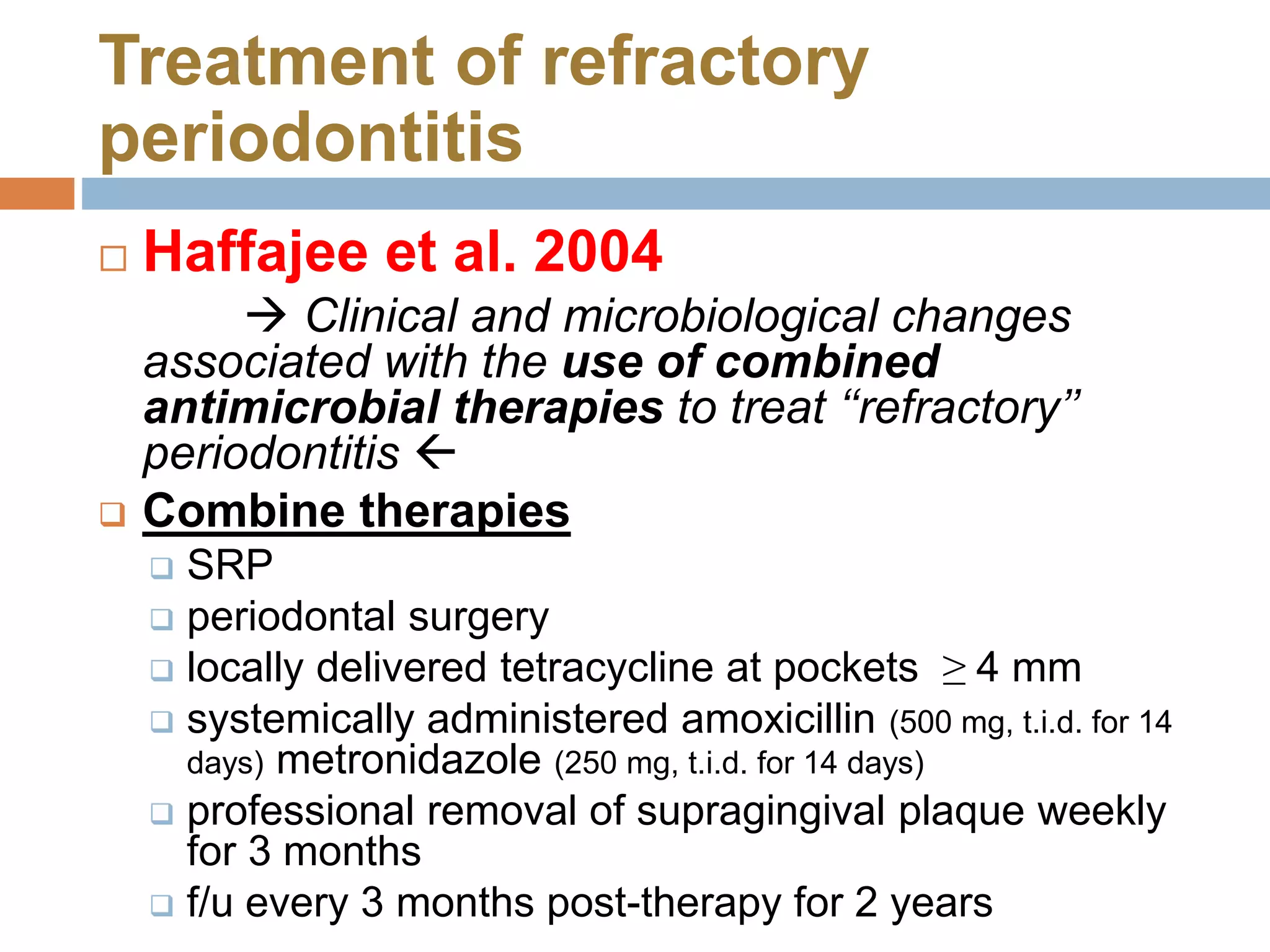
Refractory periodontitis refers to chronic periodontal disease that responds poorly to conventional treatment such as scaling and root planing. About 10-15% of patients have refractory periodontitis. Several studies examined clinical, microbiological, and immunological parameters to better diagnose and treat refractory periodontitis. One study found that levels of certain bacterial species, percentage of sites with deep pockets, and number of bacterial species with high antibody levels could predict refractory cases. Another study found elevated antibody levels to specific bacteria correlated with refractory cases. Molecular studies identified higher expression of certain genes involved in inflammation and bone resorption in refractory patients. Microarray analysis found refractory patients had persistent pathogenic bacteria after treatment. Combin