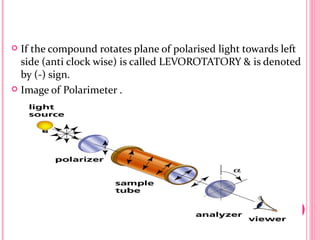
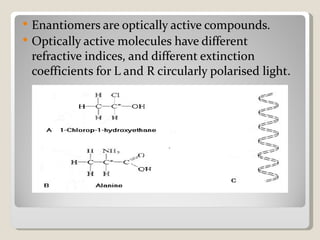
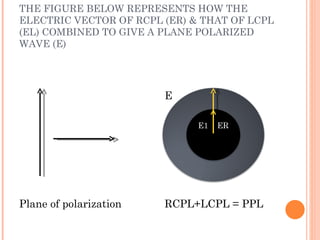
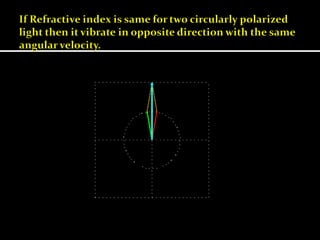
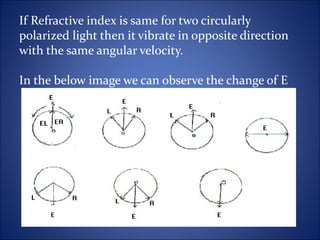
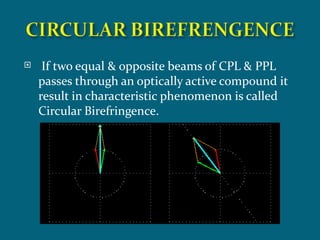
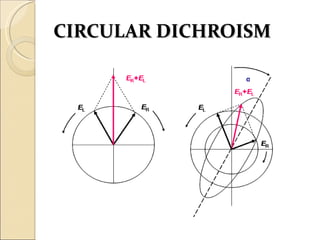
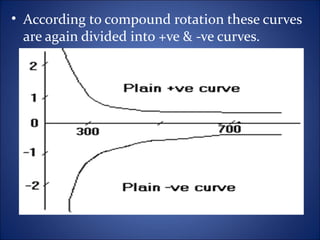
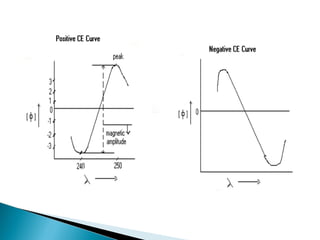
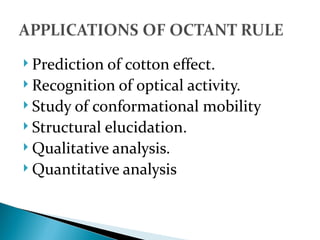
This document discusses optical rotatory dispersion (ORD), which is defined as the rate of change of specific rotation with a change in wavelength. ORD is used to determine the structures of carbonyl compounds. Key points covered include: the principles of plane polarized light, optical activity, specific rotation, and circular birefringence; factors that affect specific rotation; Cotton effects and curves; and the octant rule for establishing absolute configuration from ORD data.