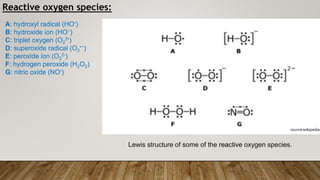
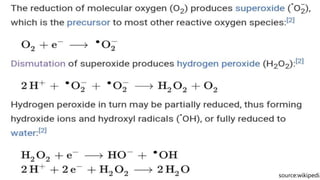
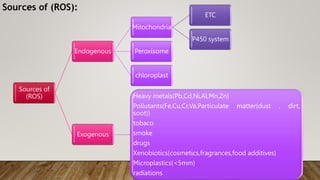
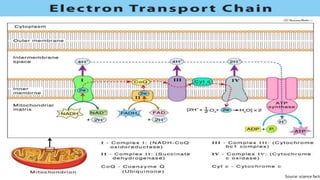
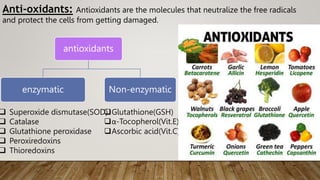
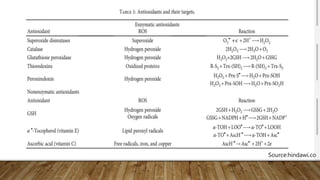
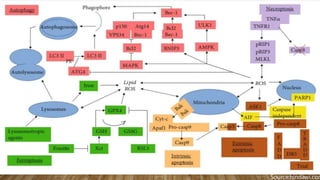
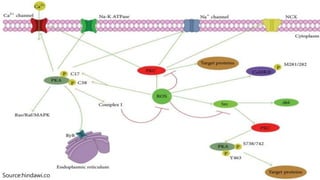
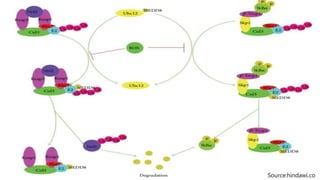
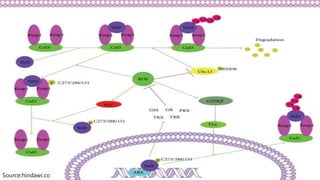
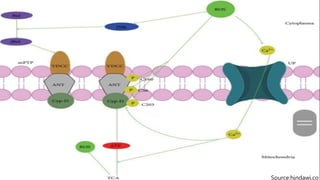
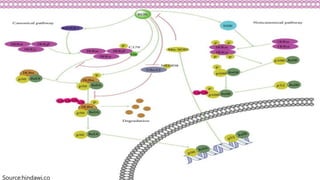
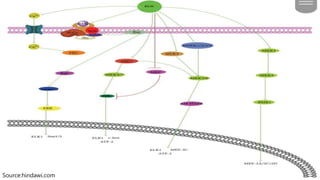
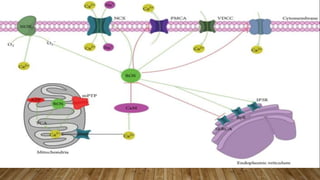
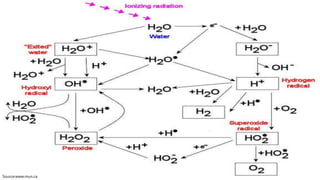
Reactive oxygen species (ROS) are highly reactive molecules formed from oxygen that can damage cells. They are produced endogenously through cellular processes like mitochondrial electron transport or immune cell activity, and exogenously through environmental exposures. At moderate levels ROS act as signaling molecules, but excess can cause oxidative stress. Antioxidants like enzymes and vitamins neutralize ROS and prevent cellular harm. The seminar discussed sources and functions of ROS as well as the protective role of antioxidants in maintaining redox balance in the body.






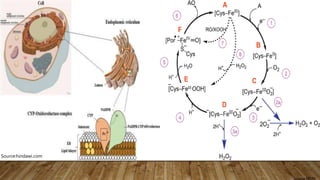
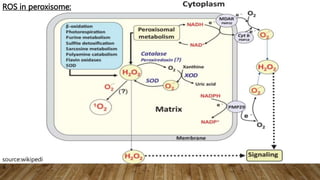
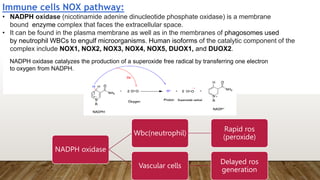


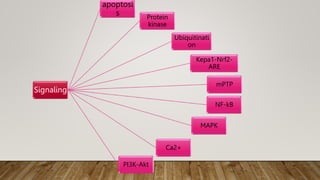






![ros [].pptx](https://image.slidesharecdn.com/rosautosaved-231017055121-dd5b0196/85/ros-pptx-34-320.jpg)