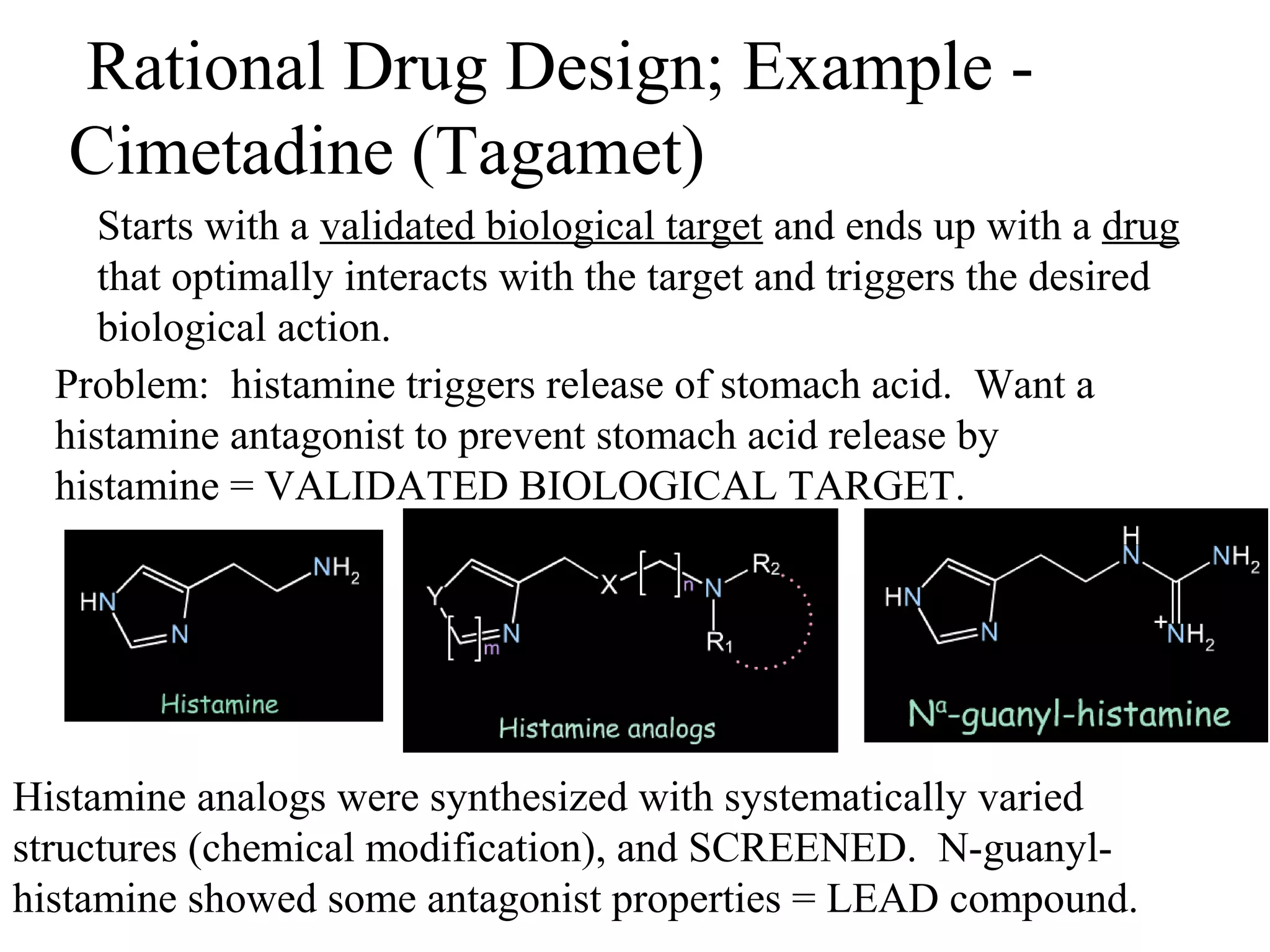
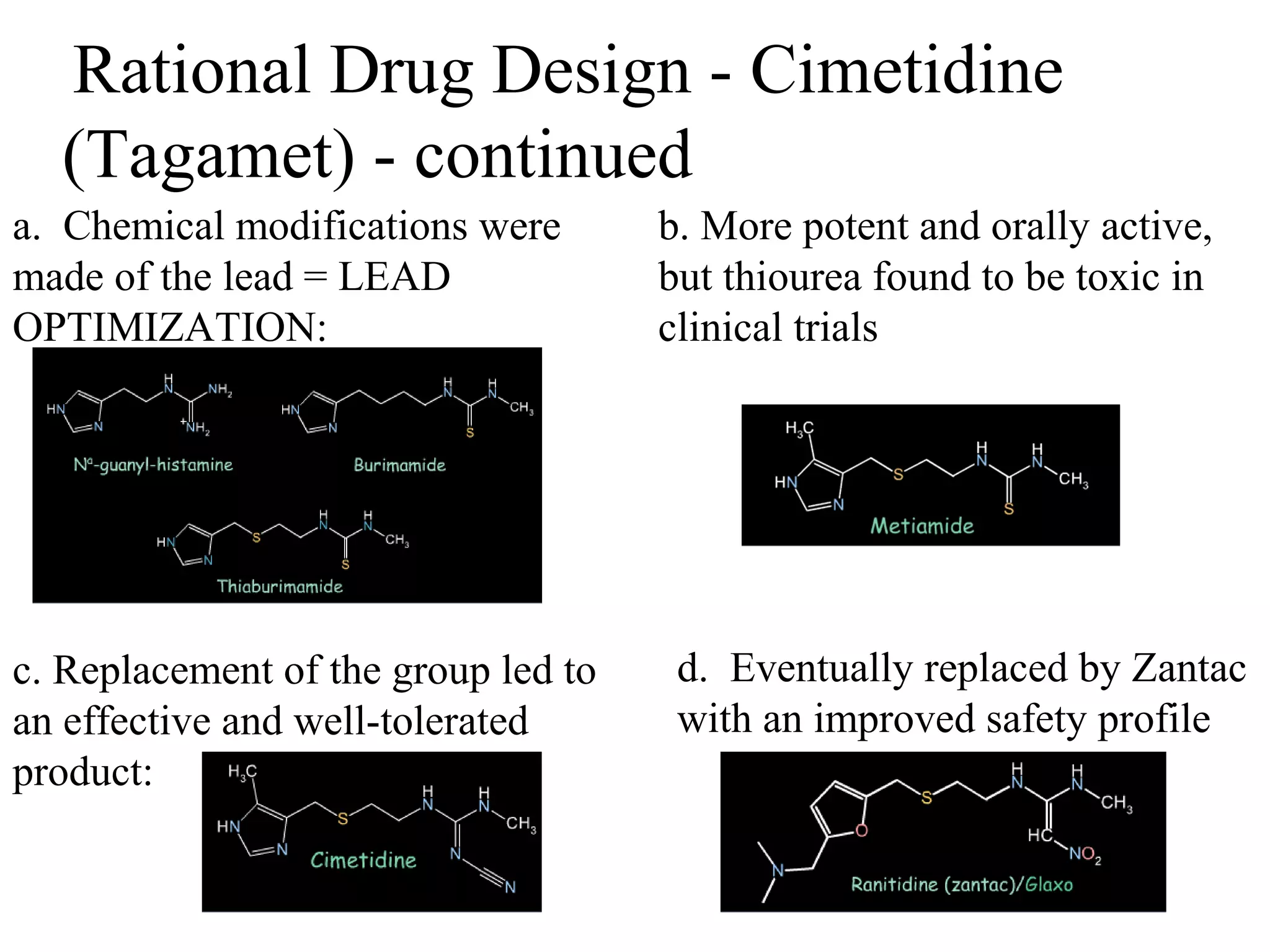
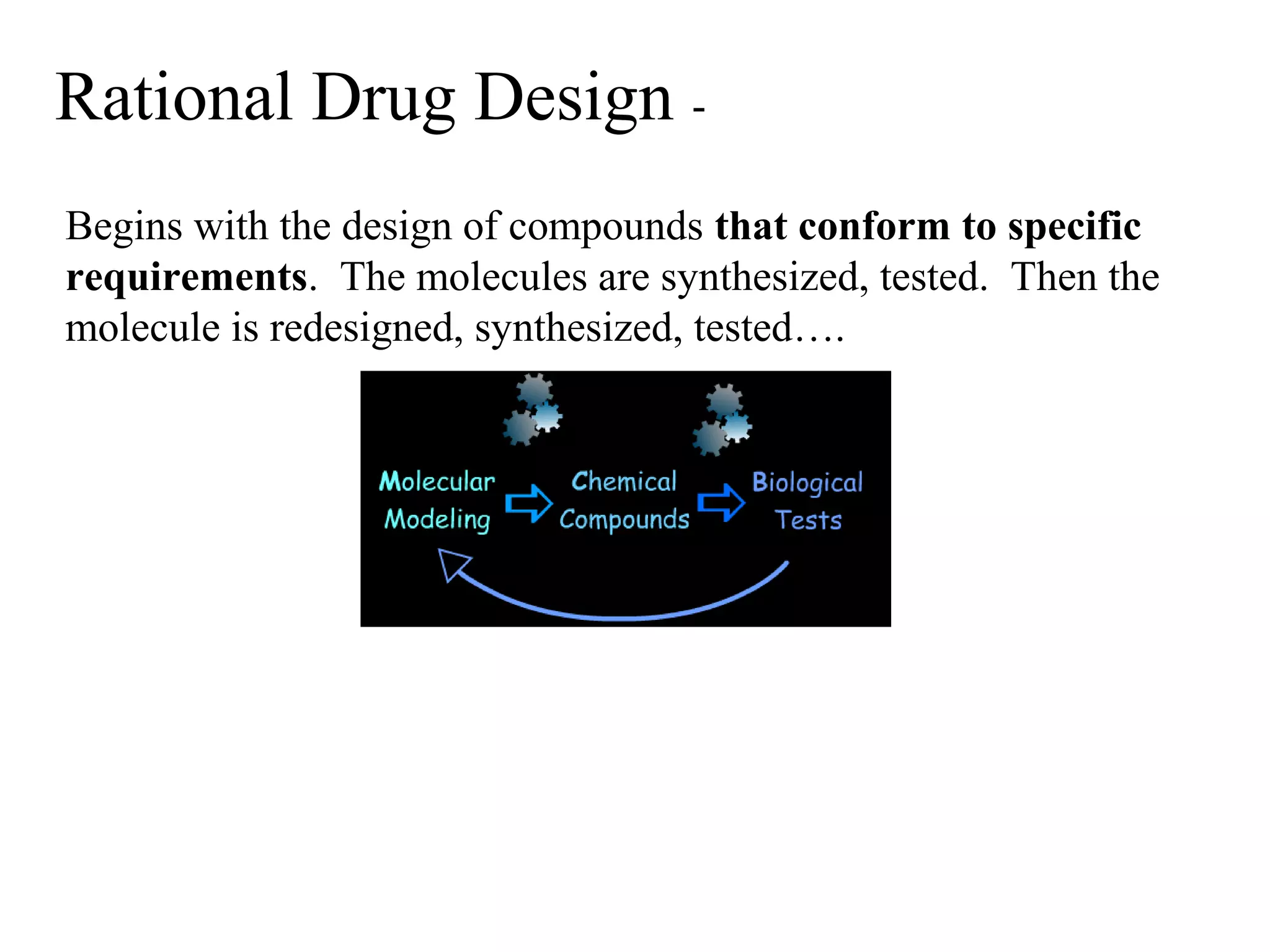
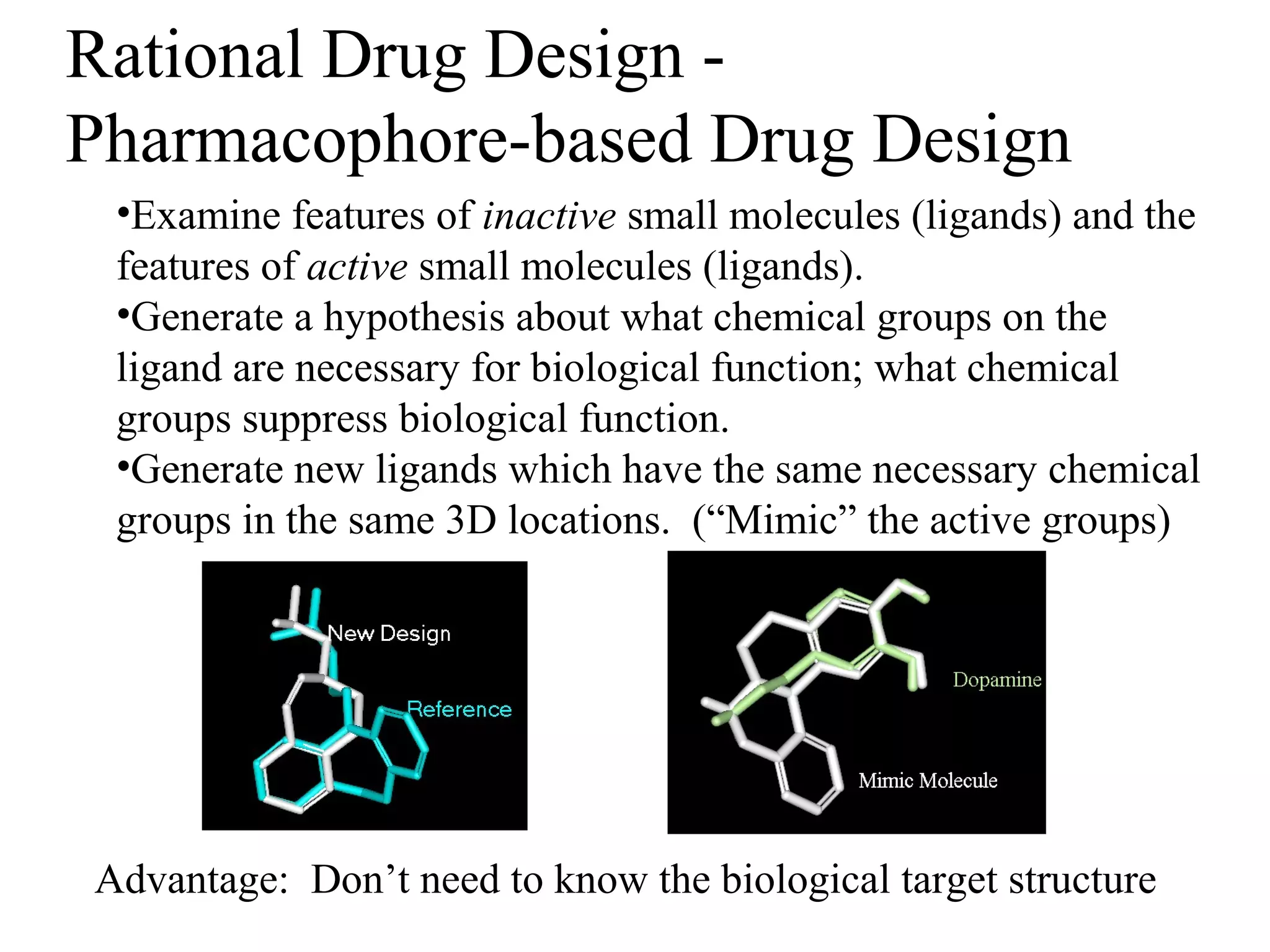
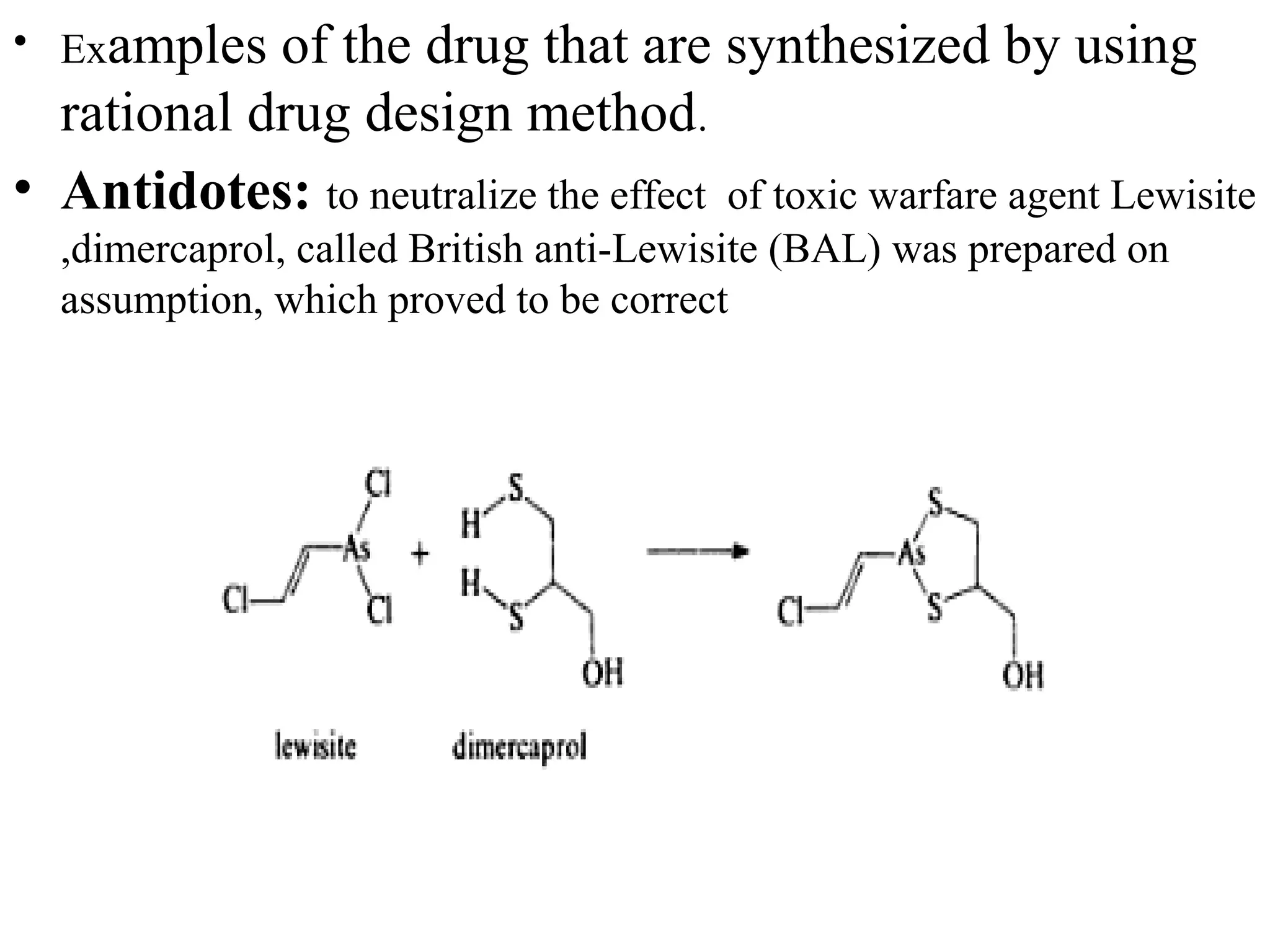
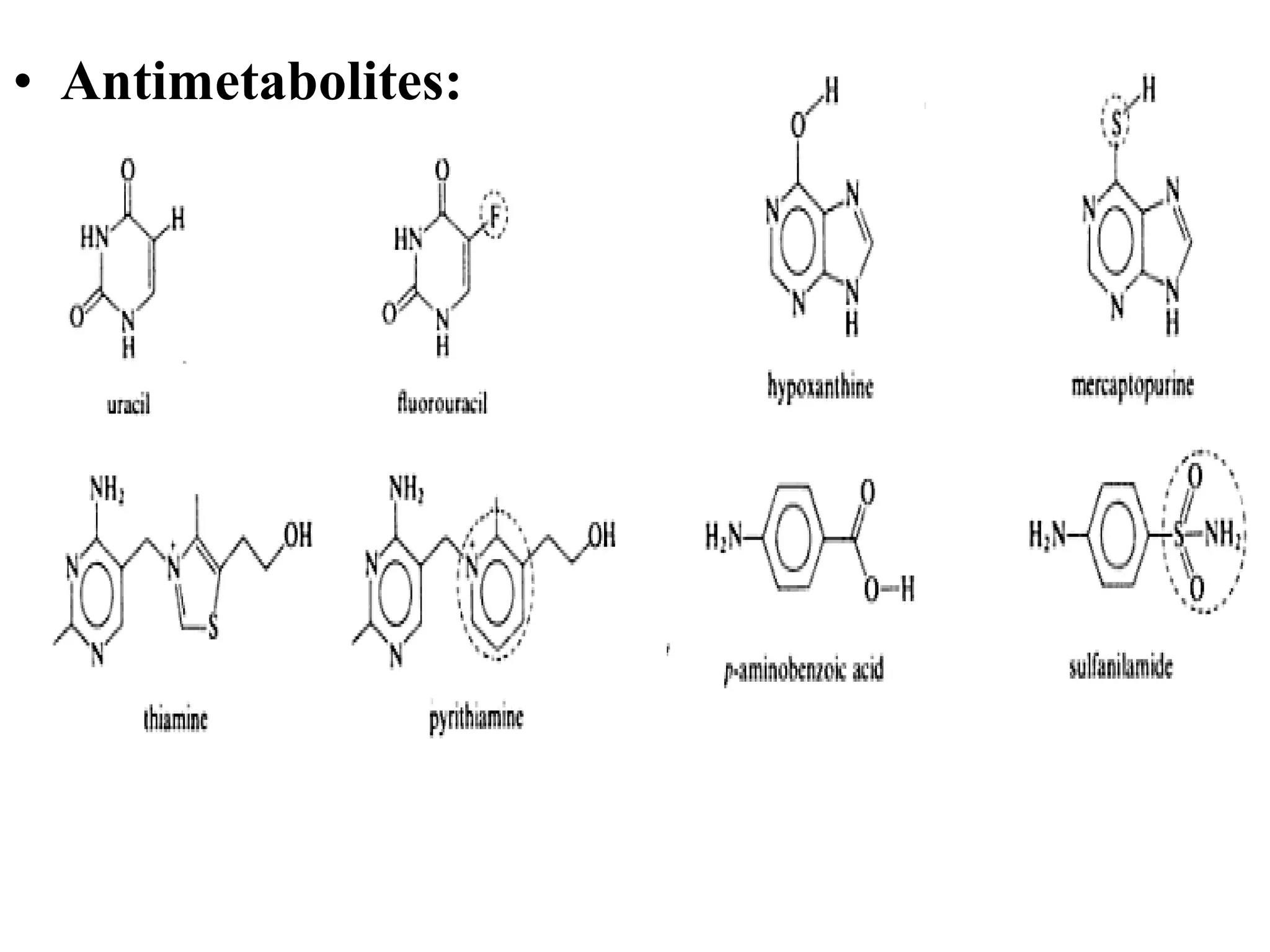
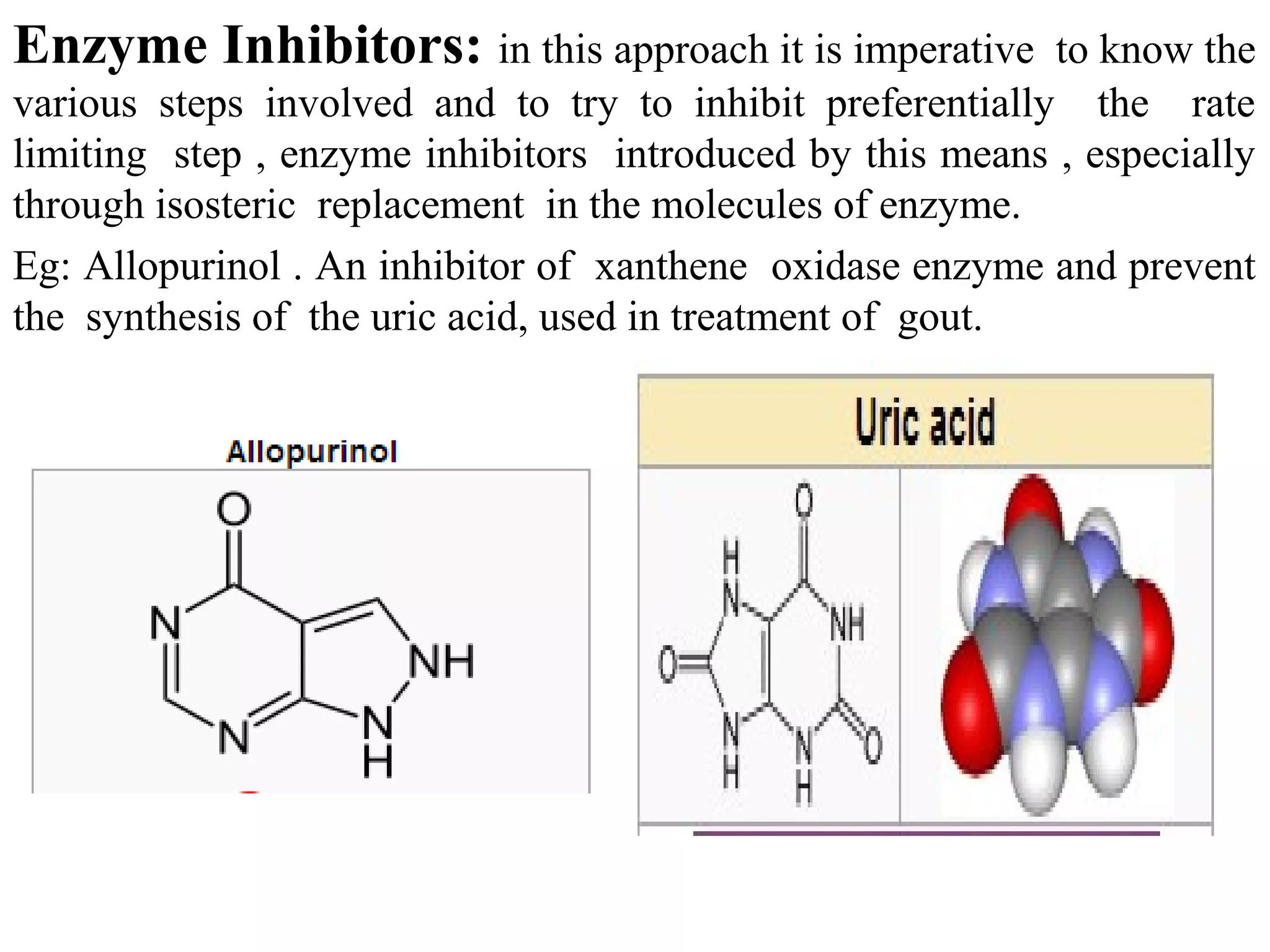
Rational drug design is a process that begins with knowledge of a biological target and aims to design small molecules that interact optimally with that target to produce a desired therapeutic effect. It involves analyzing the structures of active molecules and known targets, then designing new molecules that are predicted to specifically fit the target. This may involve modifying existing lead compounds or building new ones de novo. The goal is to develop drugs with greater potency, selectivity and fewer side effects than those found by traditional trial-and-error means. Cimetidine for reducing stomach acid is provided as an example of rational drug design, where it was optimized from an initial histamine analog lead compound.