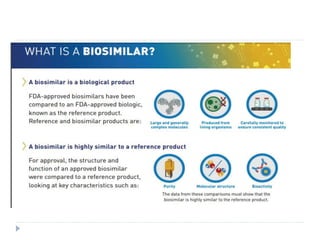
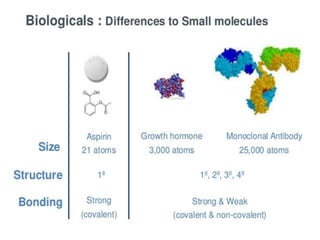
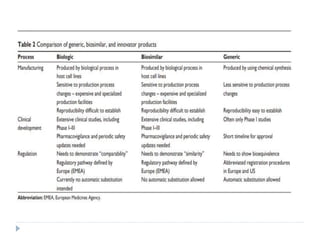
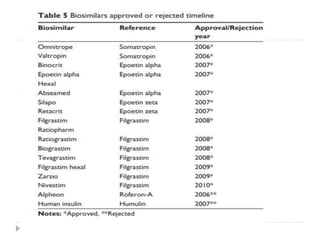
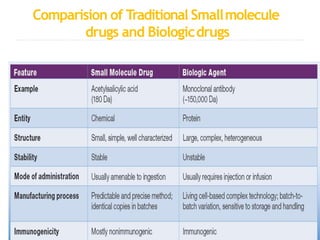
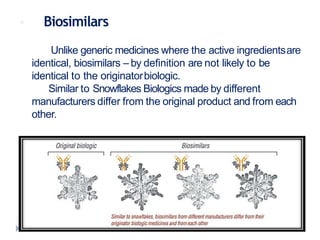
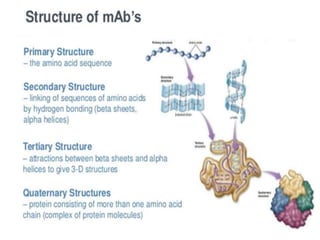
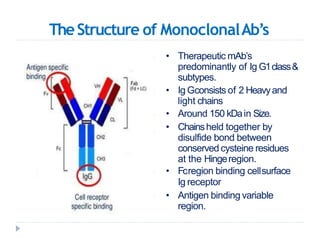
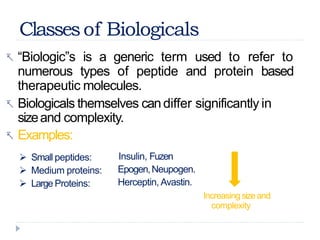
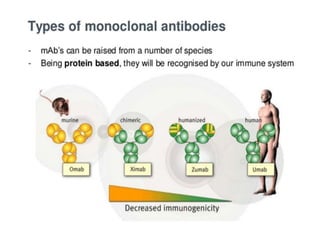
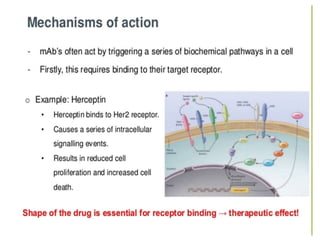
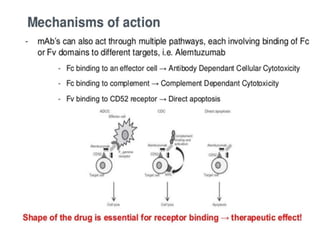
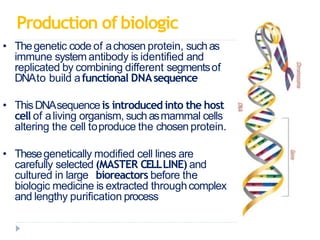
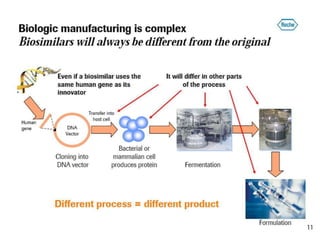
Biosimilars are biologic medicines that are developed to be similar to existing approved biologic medicines known as reference medicines. Biosimilars must demonstrate similarity to the reference medicine in terms of quality, safety and efficacy through comprehensive testing and analysis. While biosimilars may provide reduced costs and increased access to biologic treatments, they are more complex than traditional small molecule drugs due to differences in size, structure, manufacturing processes, and potential for immunogenicity. Thorough evaluation and regulation is required to ensure biosimilars are interchangeable for the reference product without compromising patient safety.