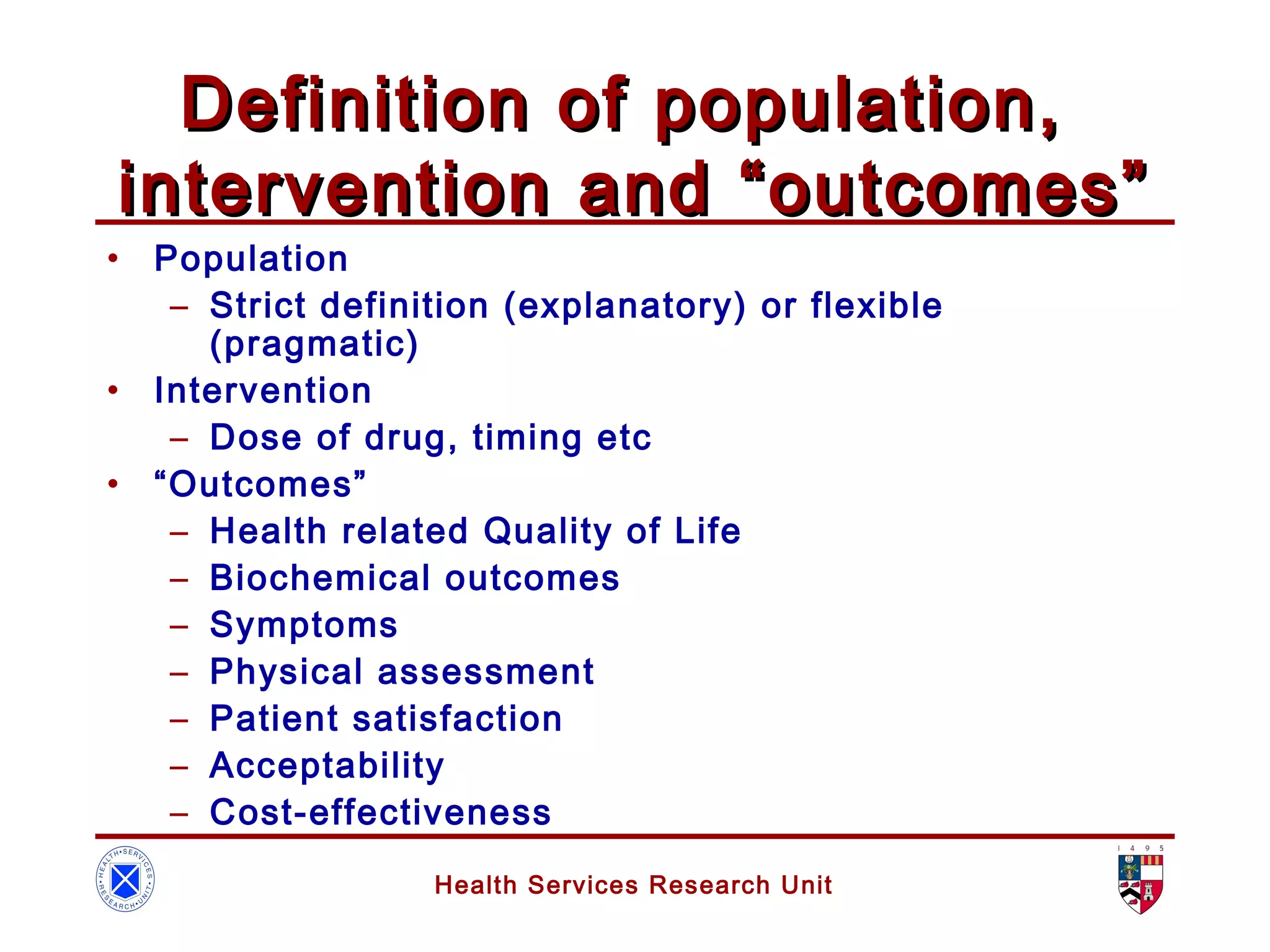
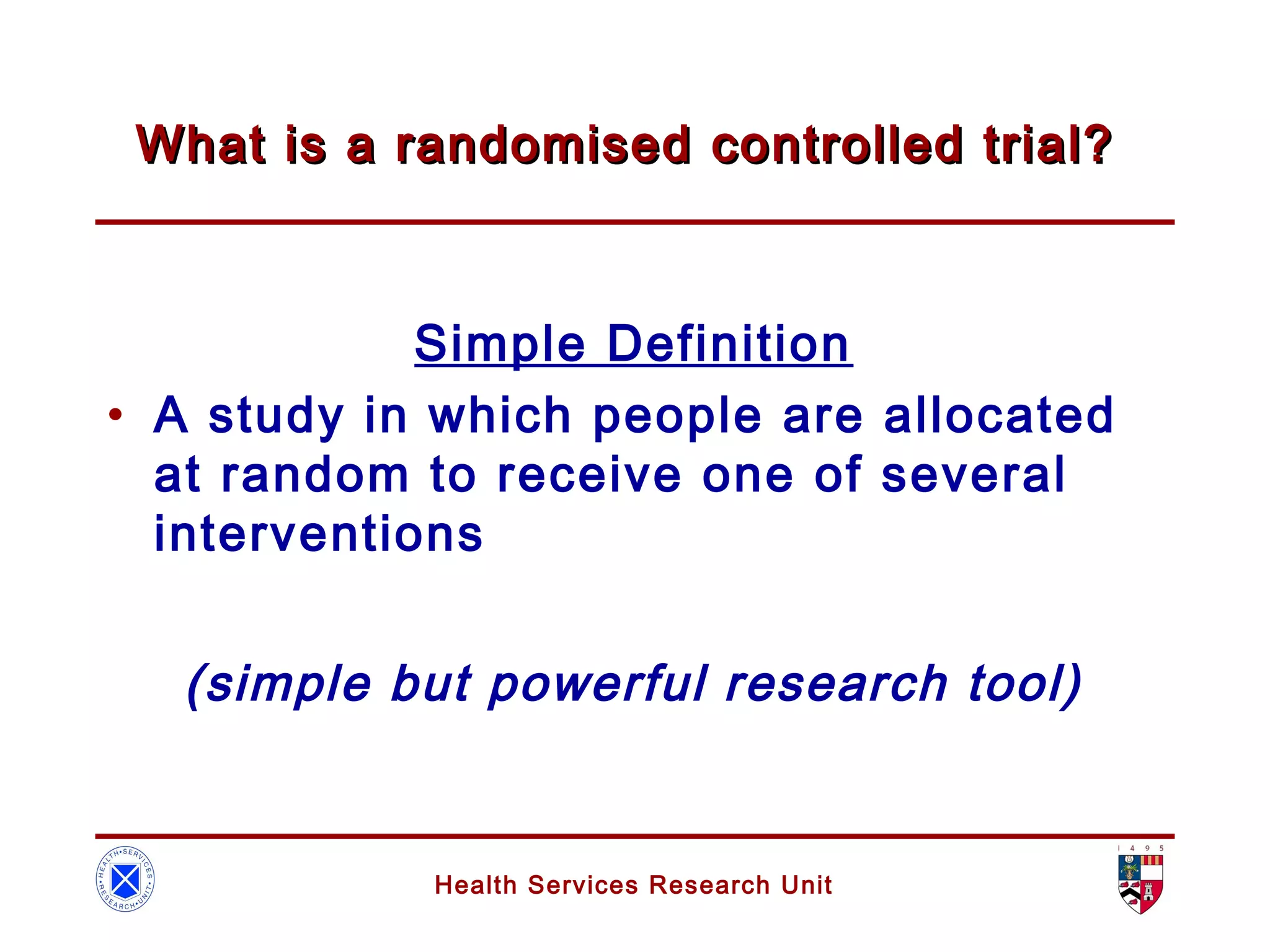
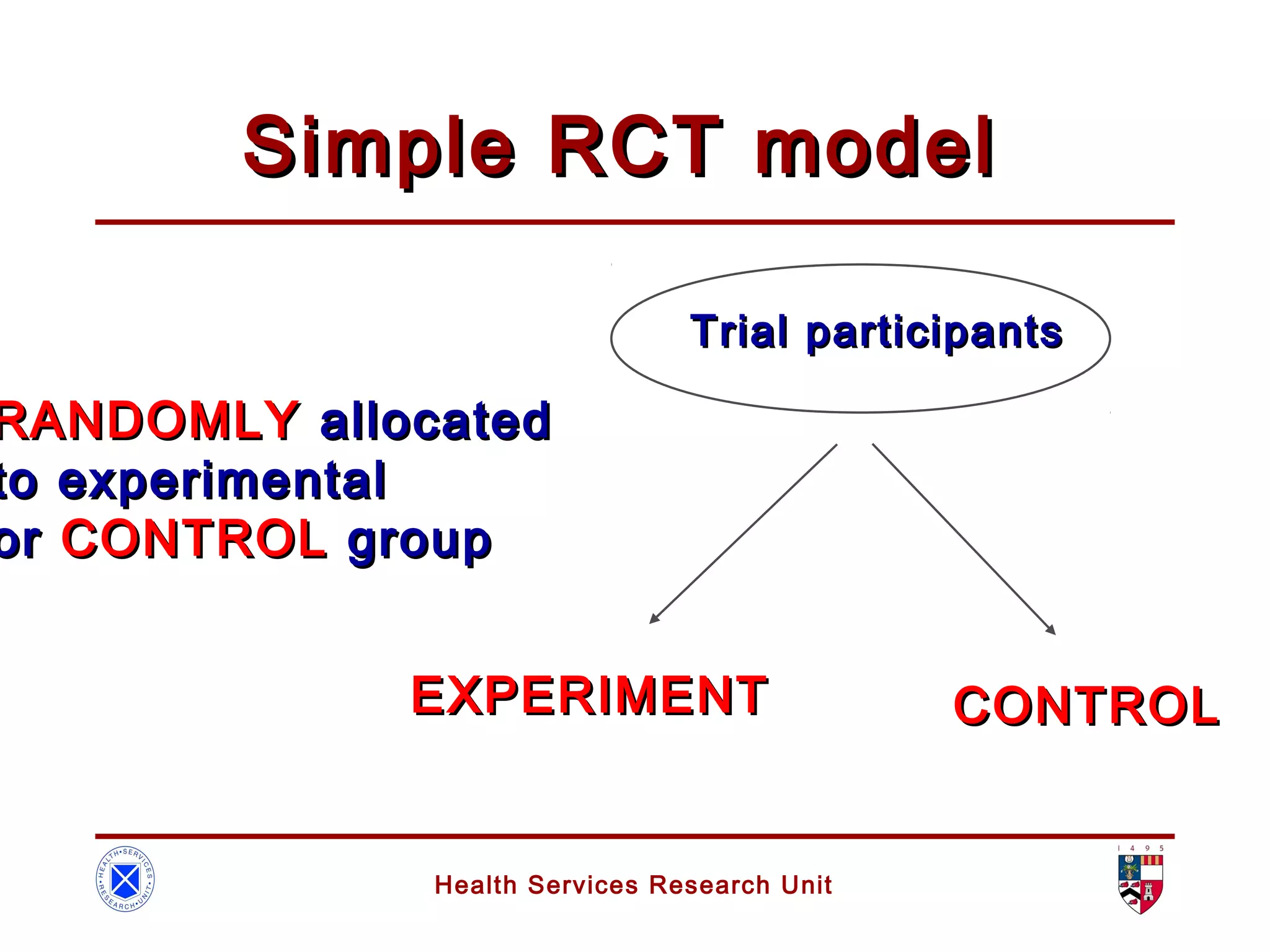
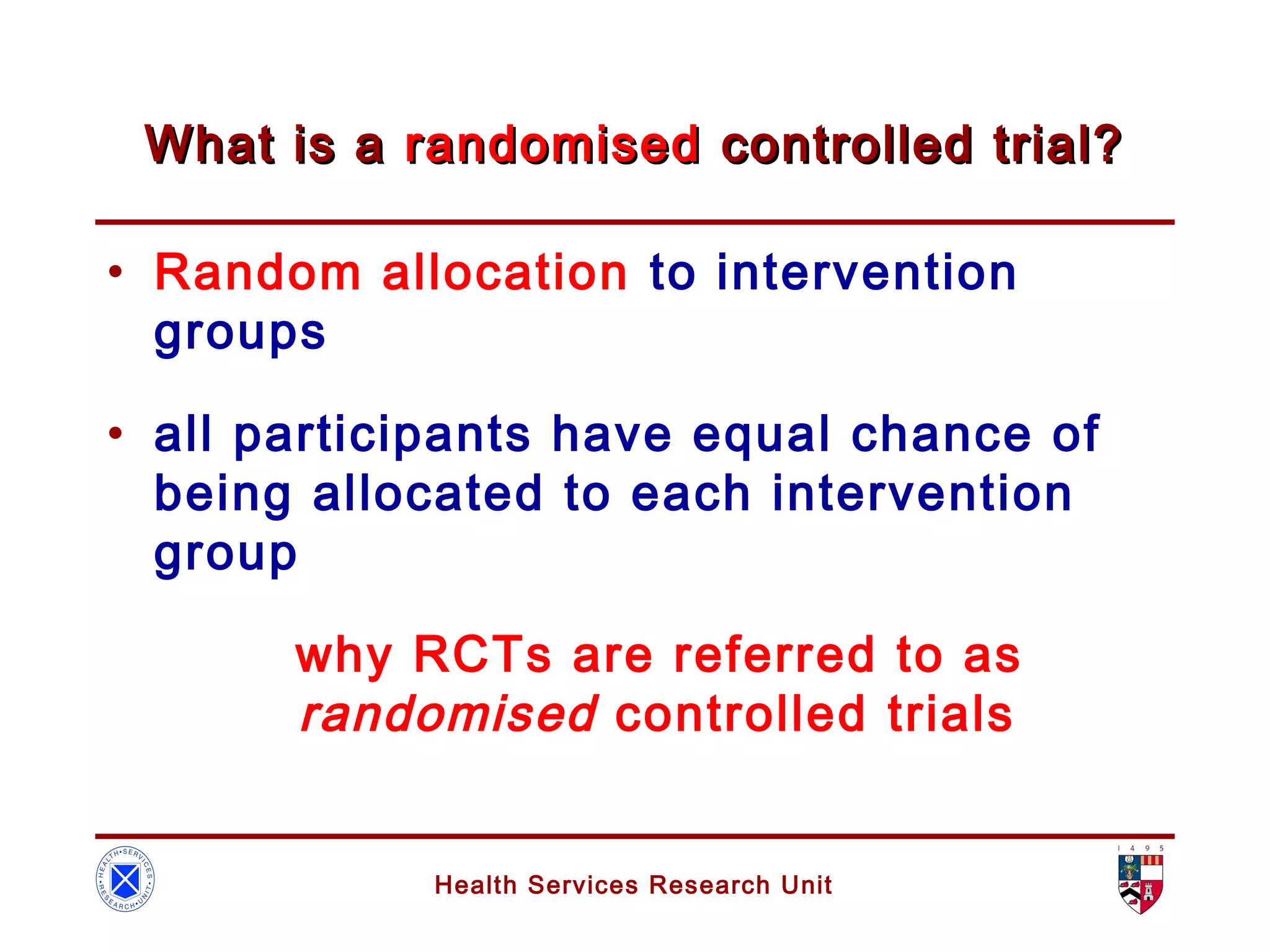
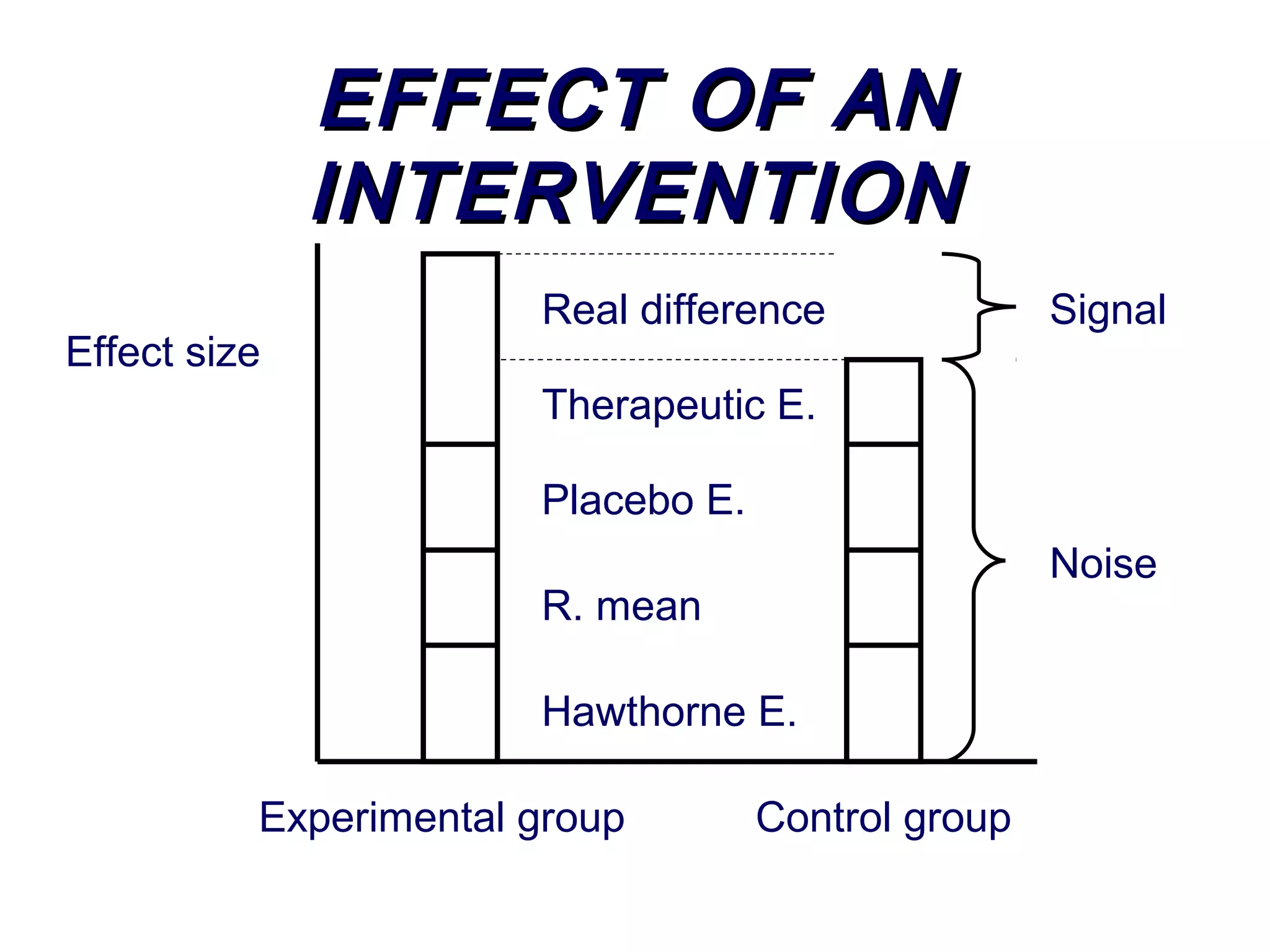
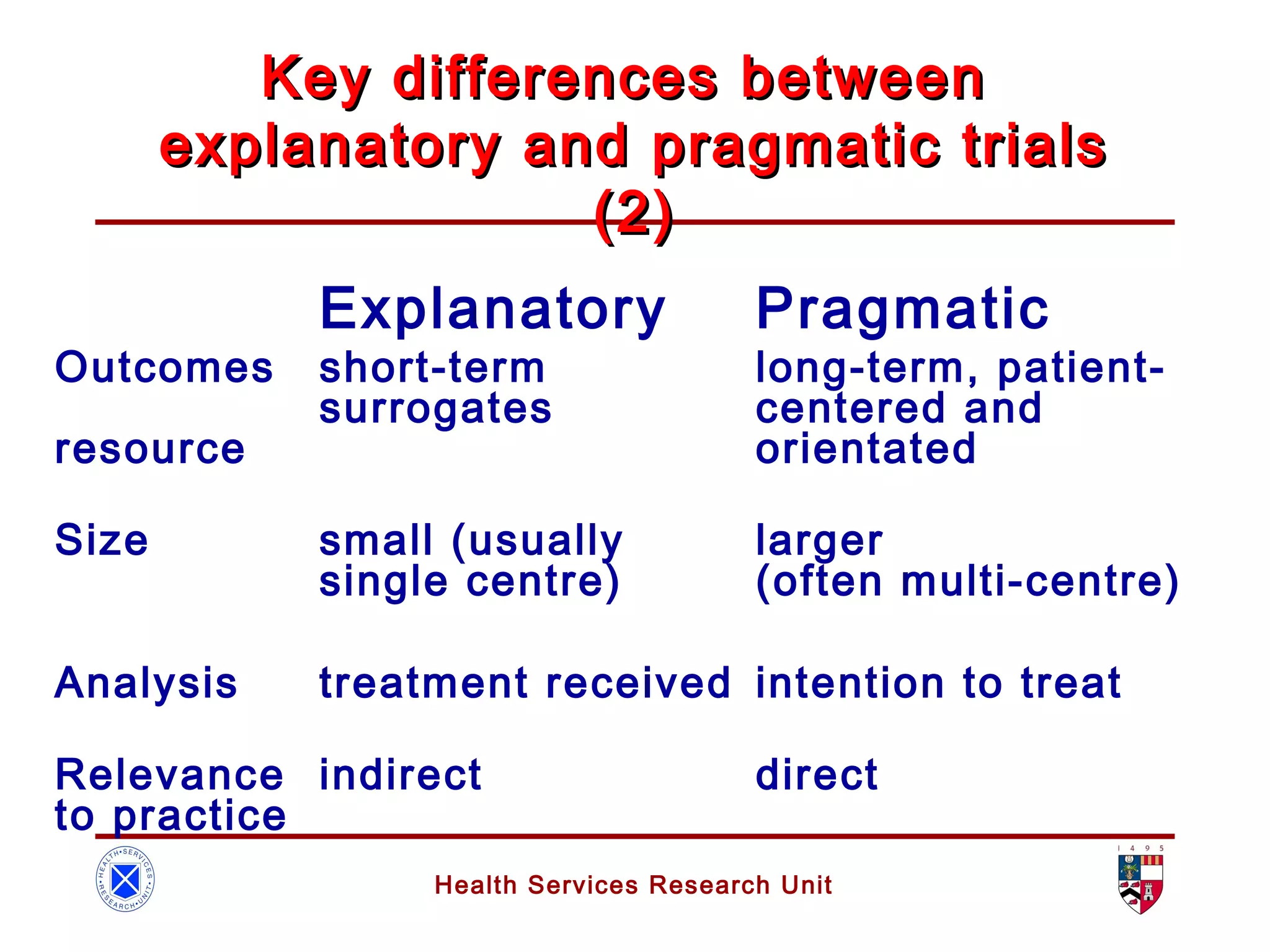
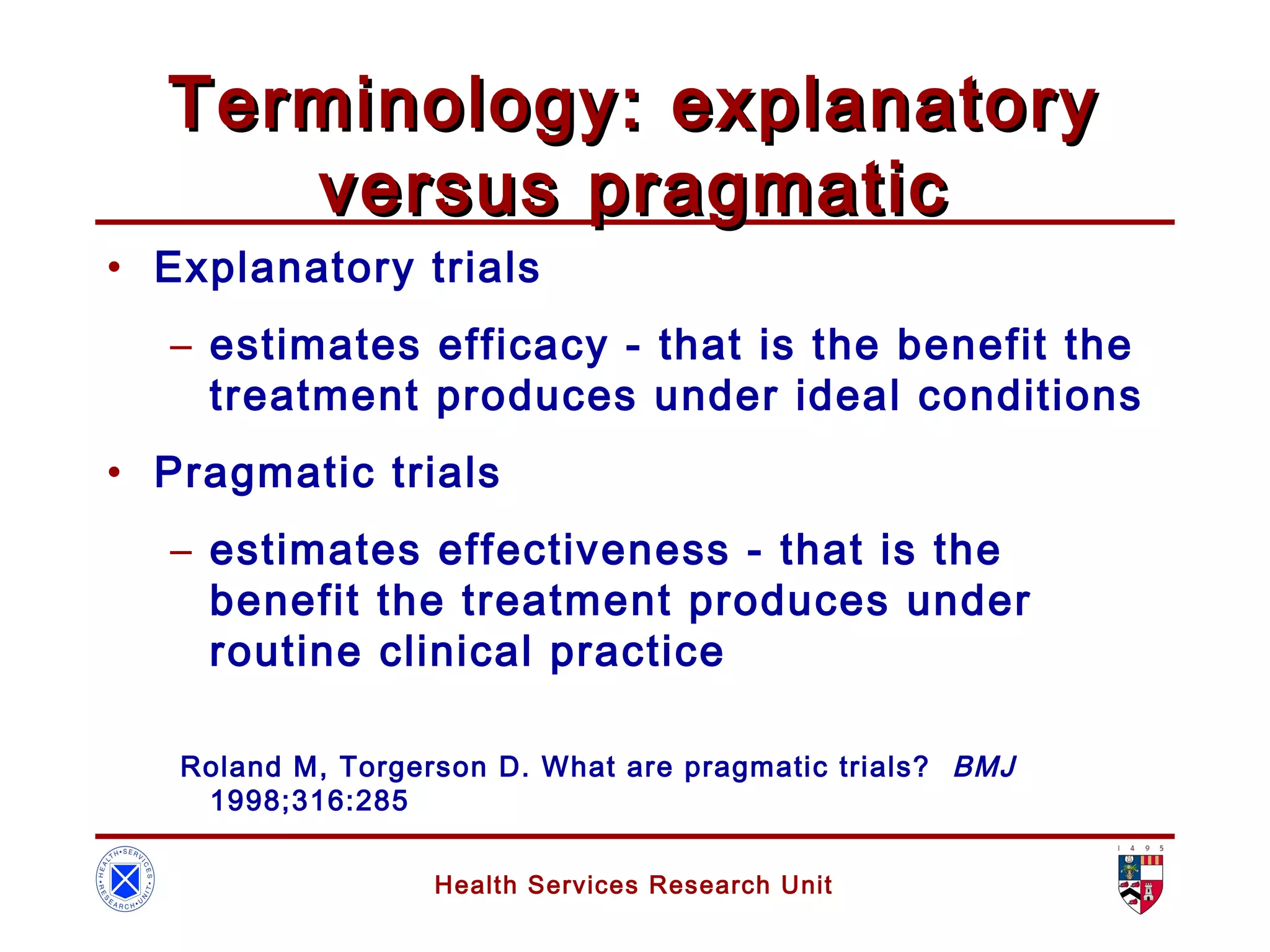
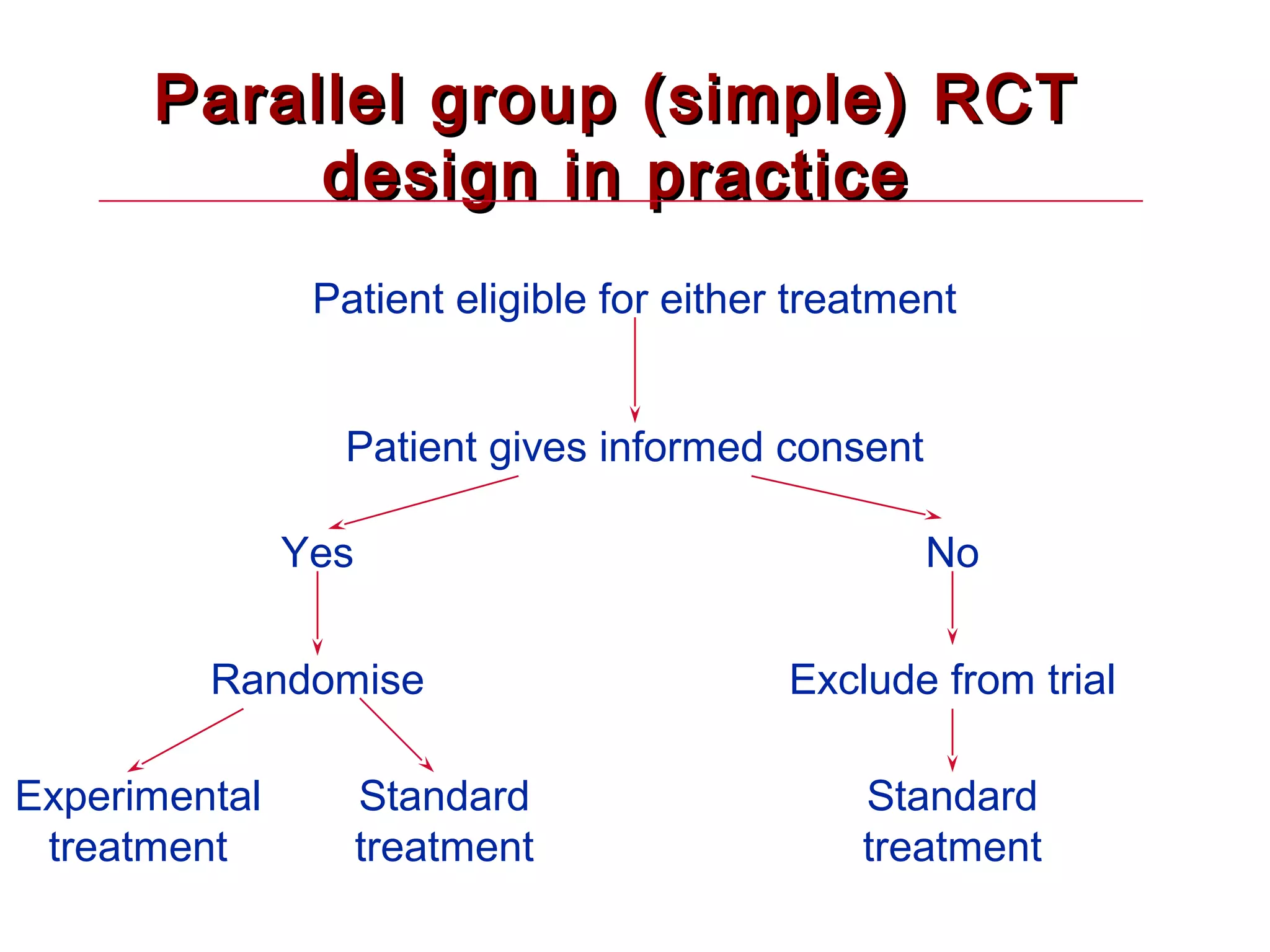
The document discusses randomized controlled trials (RCTs). It summarizes that HSRU is funded by the Scottish Government Health Directorates, and the author accepts full responsibility for the talk. RCTs are the gold standard for evaluating healthcare interventions through random allocation of participants to experimental or control groups in order to minimize bias.