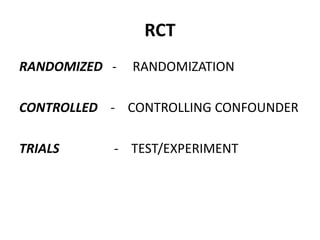
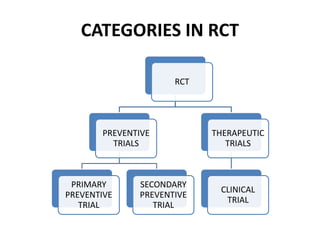
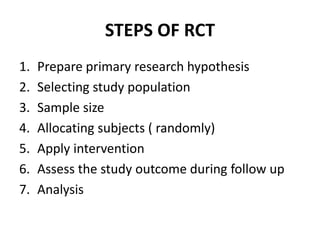
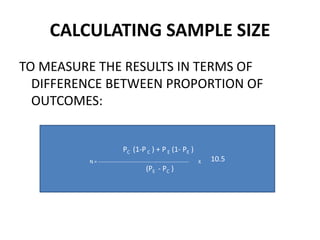
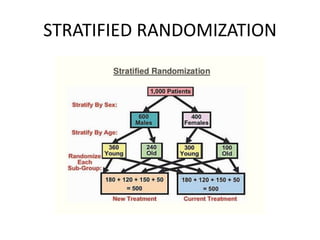
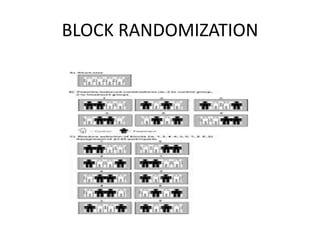
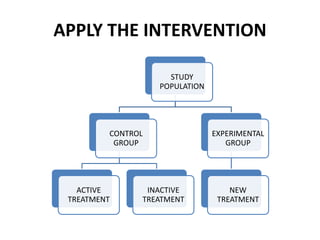
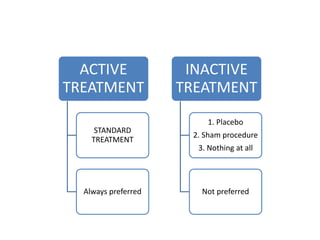
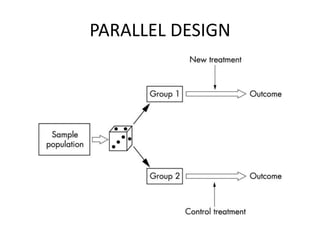
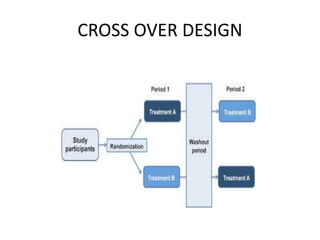
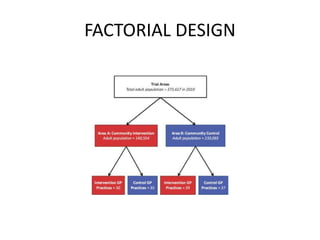
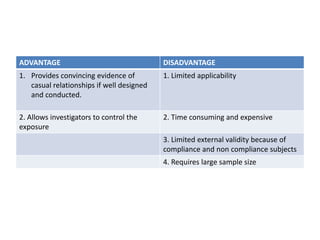
This document provides an overview of randomized controlled trials (RCTs). It defines RCTs as planned experiments where individuals are randomly assigned to experimental and control groups to assess the effect of a preventive or therapeutic measure. RCTs are considered the gold standard for epidemiological studies as they can provide the strongest evidence of causation. The document outlines the key aspects of RCTs, including categories (preventive and therapeutic trials), steps (from developing hypotheses to analysis), ethical considerations, randomization techniques, blinding/masking, and uses. RCTs aim to control for confounding factors and minimize bias through random assignment and blinding.