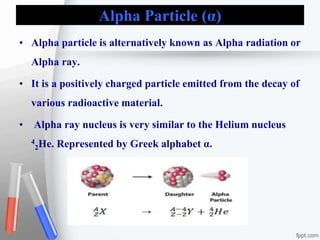
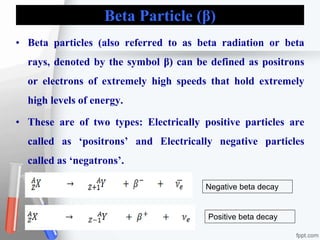
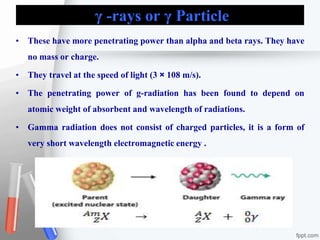
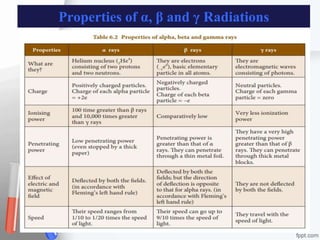
The document provides an overview of radiopharmaceuticals and radioactivity, including the types of radiation (alpha, beta, and gamma) and their properties. It discusses the half-life of radioactive substances, units of radioactivity, and various pharmaceutical applications, notably in cancer and thyroid treatment. Additionally, it outlines safety precautions for handling radioactive substances and the importance of proper storage and hygiene practices.