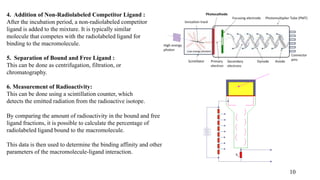
The document discusses radiometric titrations and radio-release methods, which utilize radioactive isotopes to measure analyte concentrations and binding interactions respectively. It details various techniques and principles for each method, highlighting their applications in drug discovery and biochemical studies. The document also addresses advantages, disadvantages, and specialized procedures necessary for these radiochemical techniques.