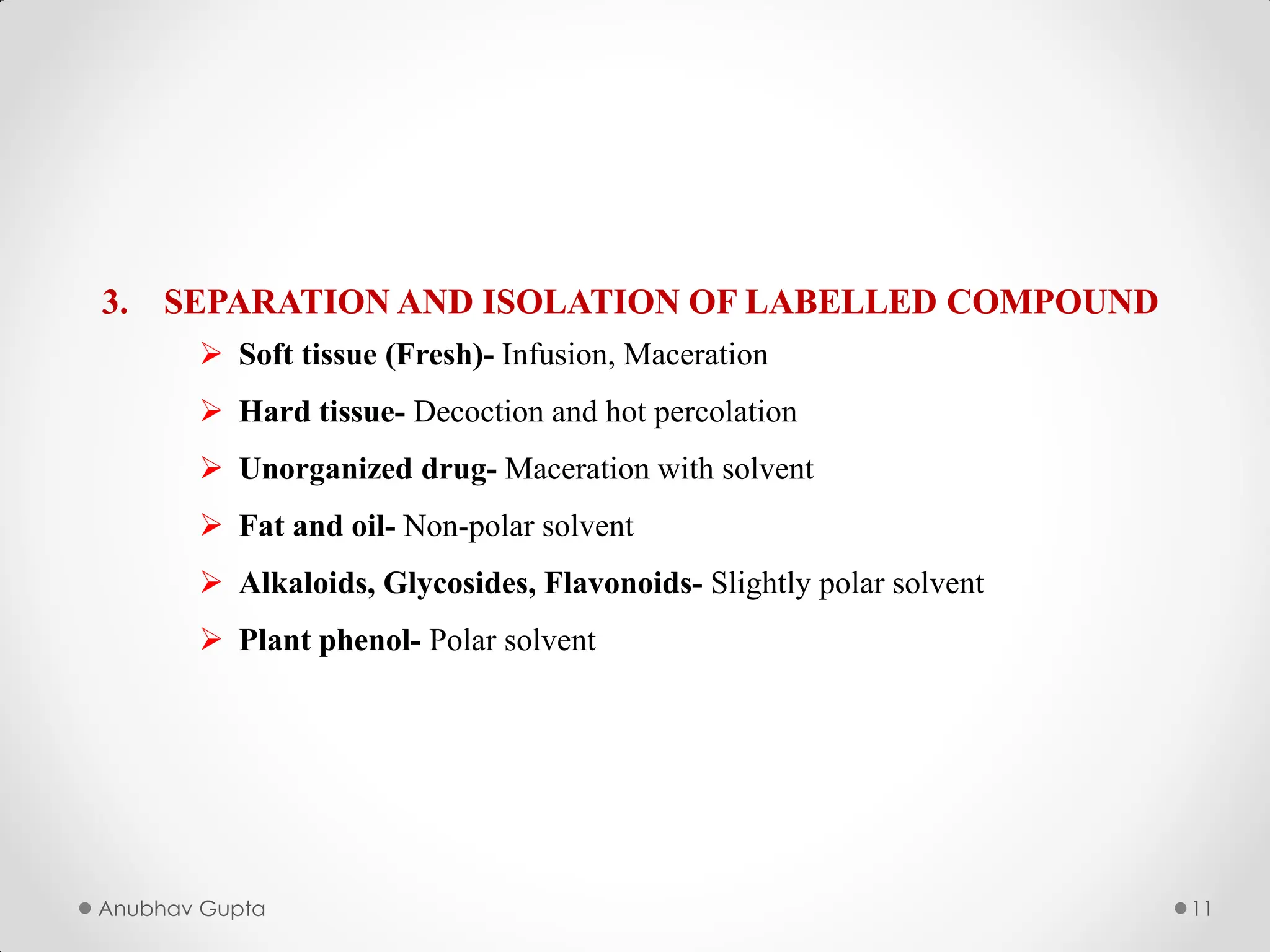
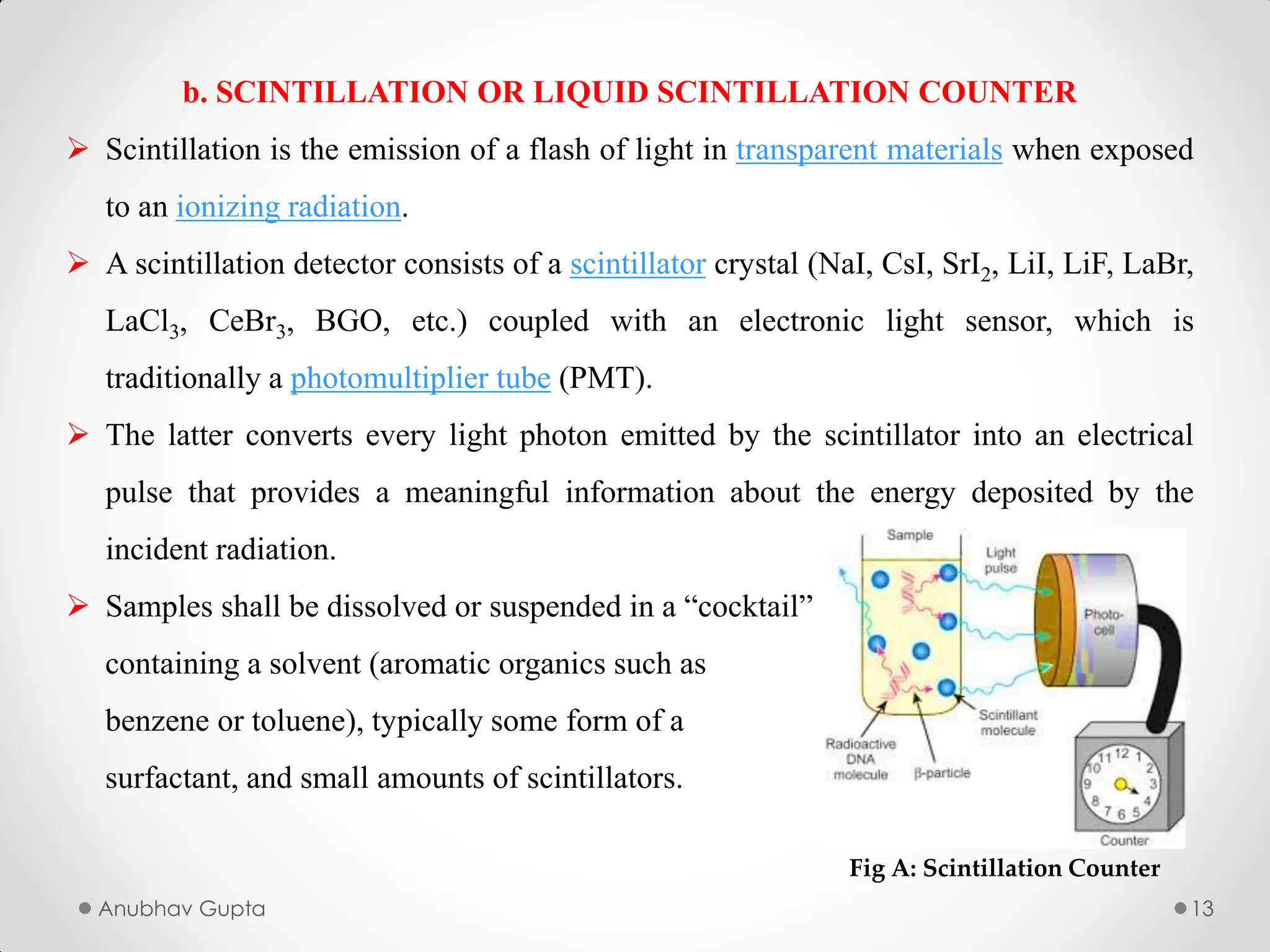
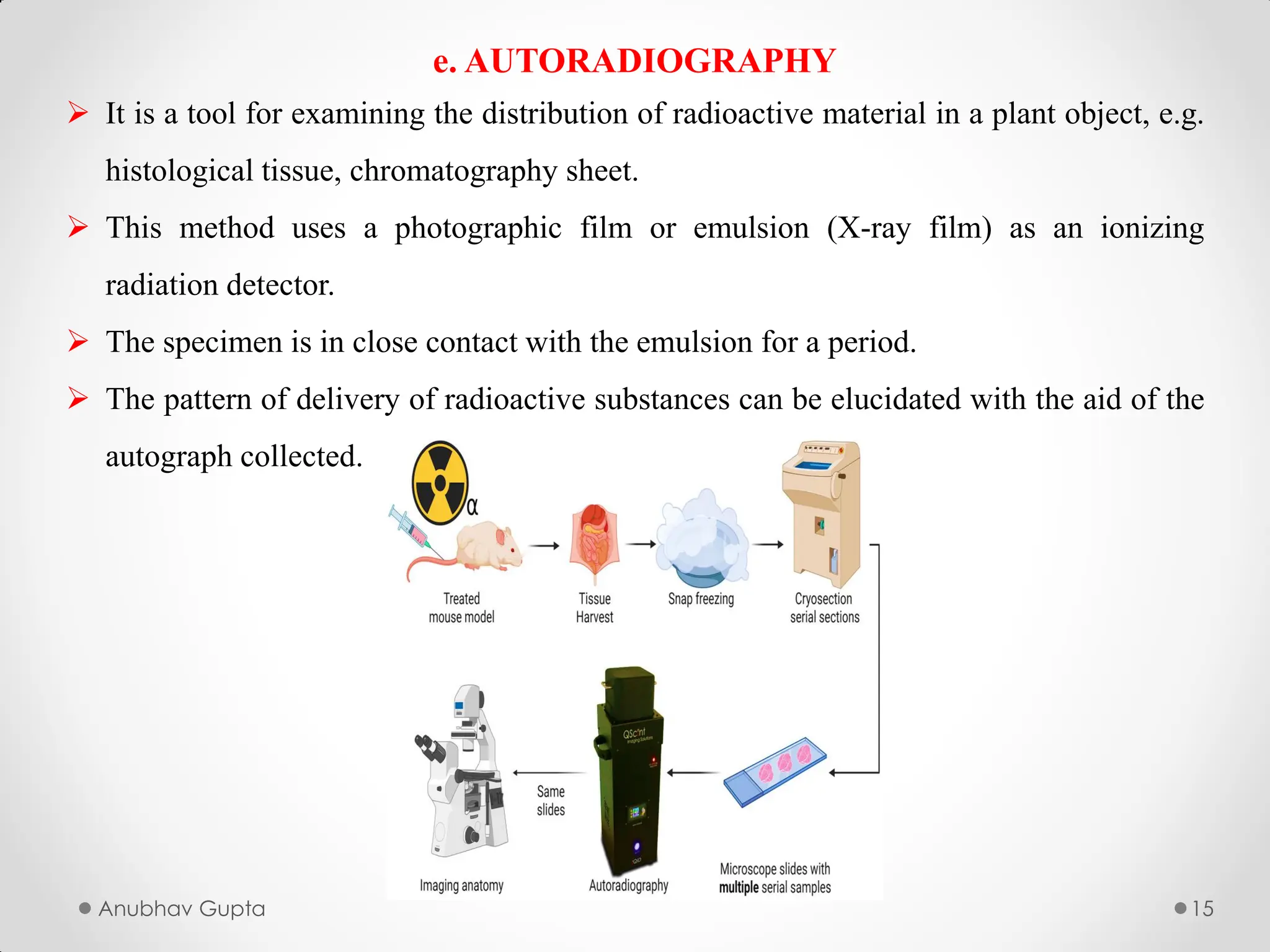
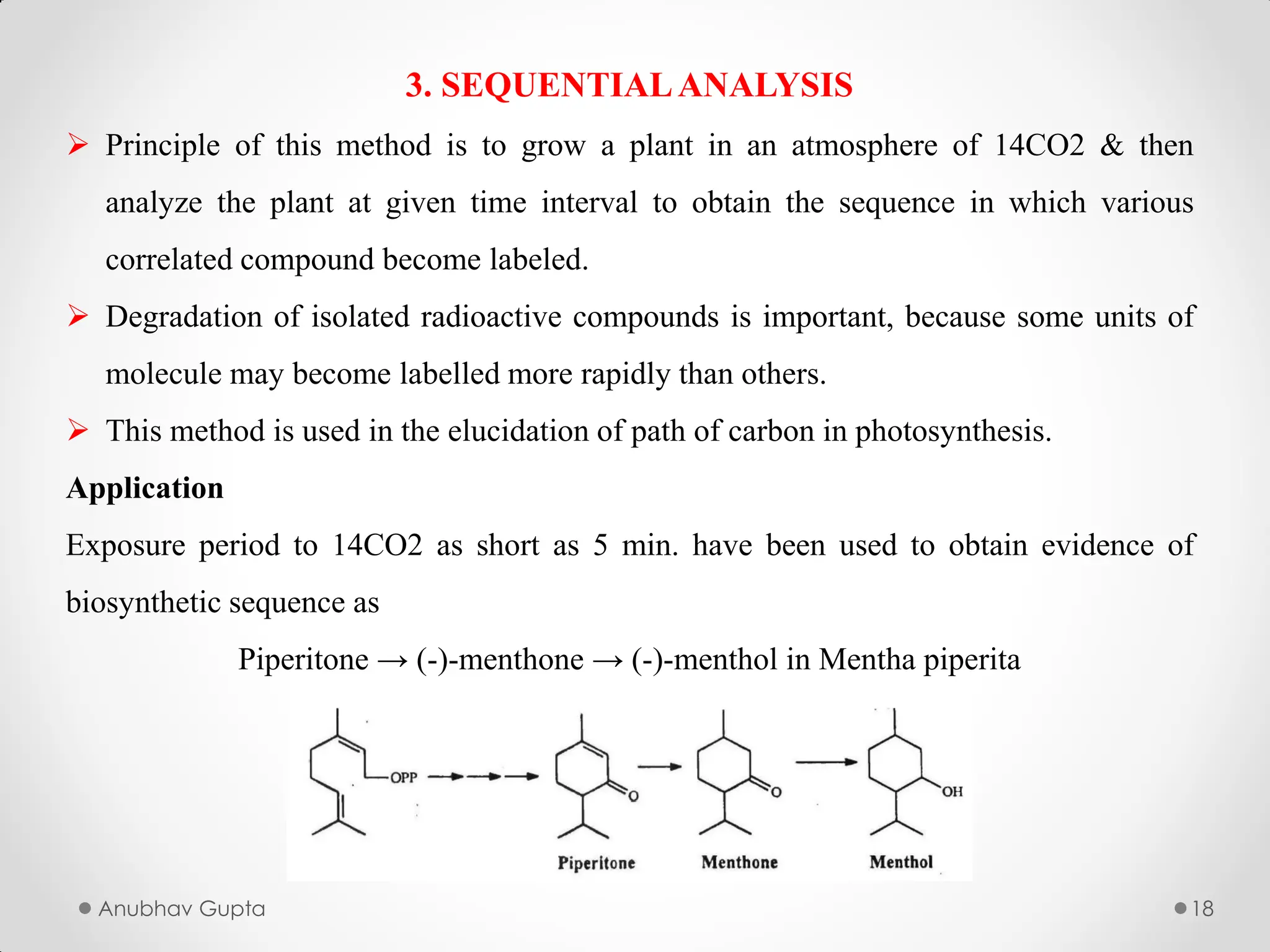
In this we will study about the utilization of radioactive isotypes in biogenesis, alongwith the utilization of Tracer Compounds.
Prepared By:
Anubhav Gupta
Asst. Professor
Ashoka Institute of Technology and Management Varanasi
References:
Wikipedia & Web