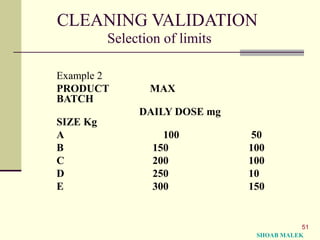
This document provides background information and guidance on cleaning validation for pharmaceutical manufacturing. It discusses how the FDA began requiring validation of cleaning processes in the 1960s after issues with penicillin contamination. The document defines cleaning validation and outlines cGMP requirements. It provides guidance on general requirements including conducting multiple successful cleaning cycles, evaluating equipment cleaning strategies, and setting validation limits. It also discusses considerations for evaluating cleaning processes and detecting residues.