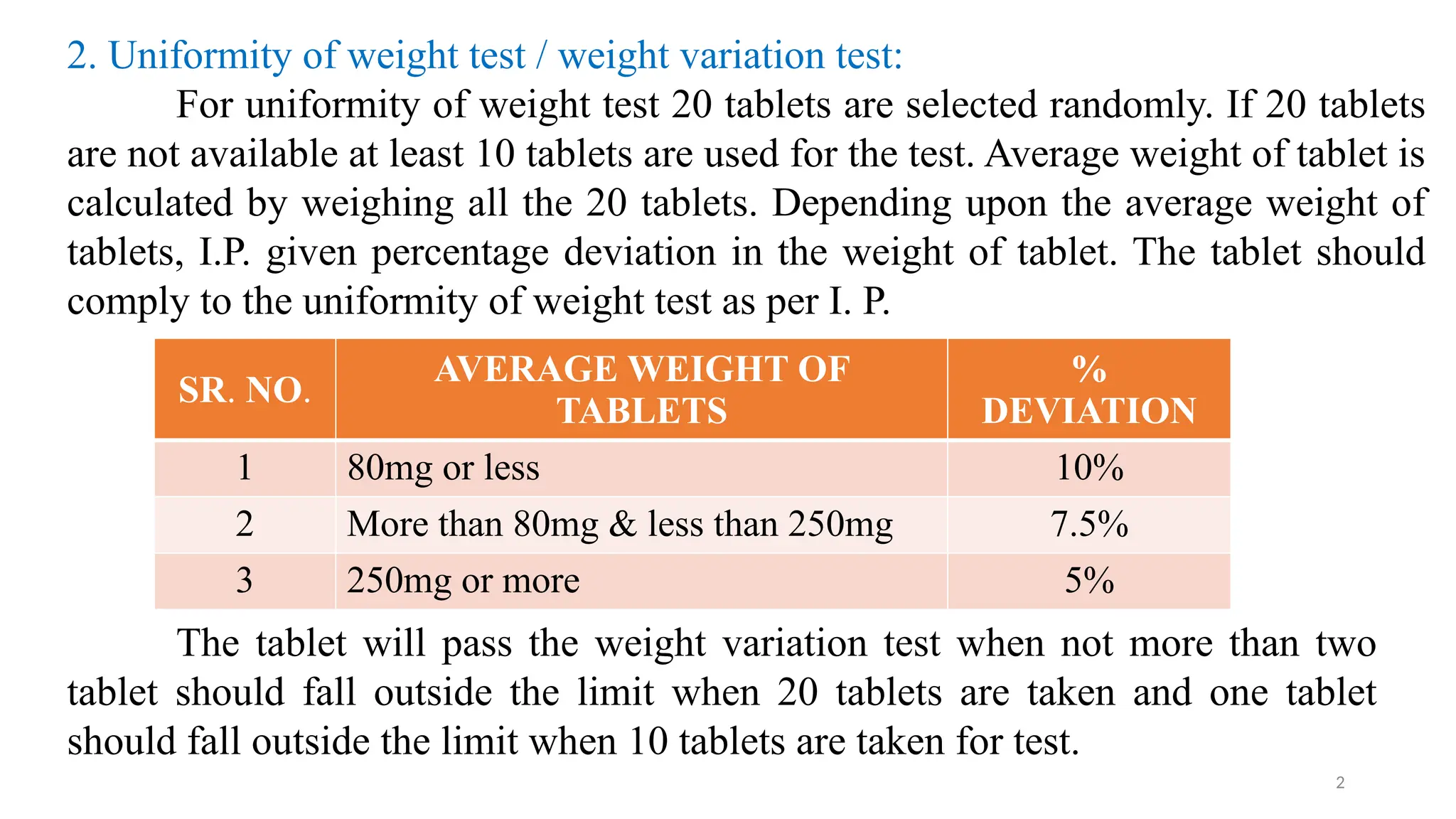
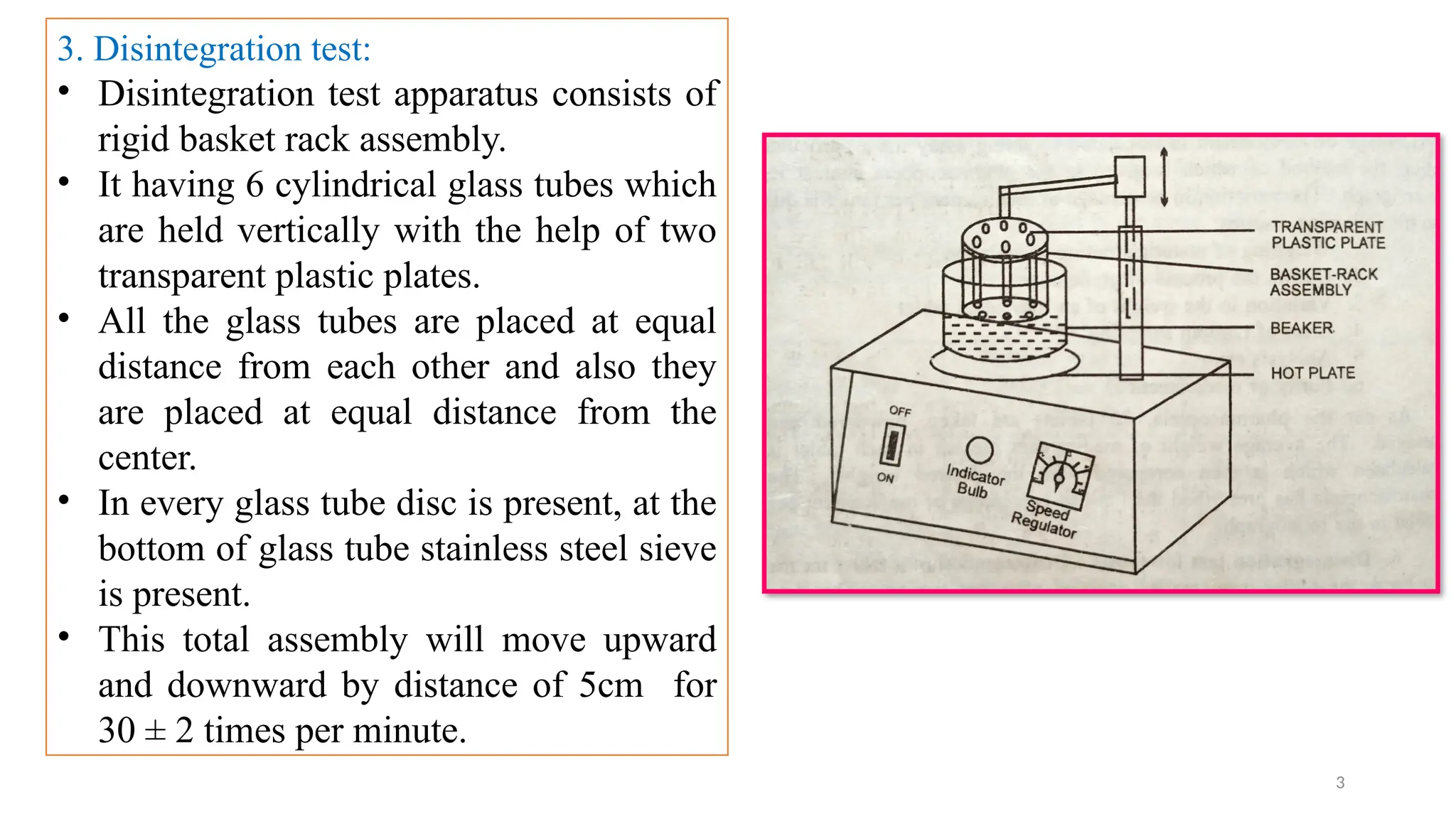
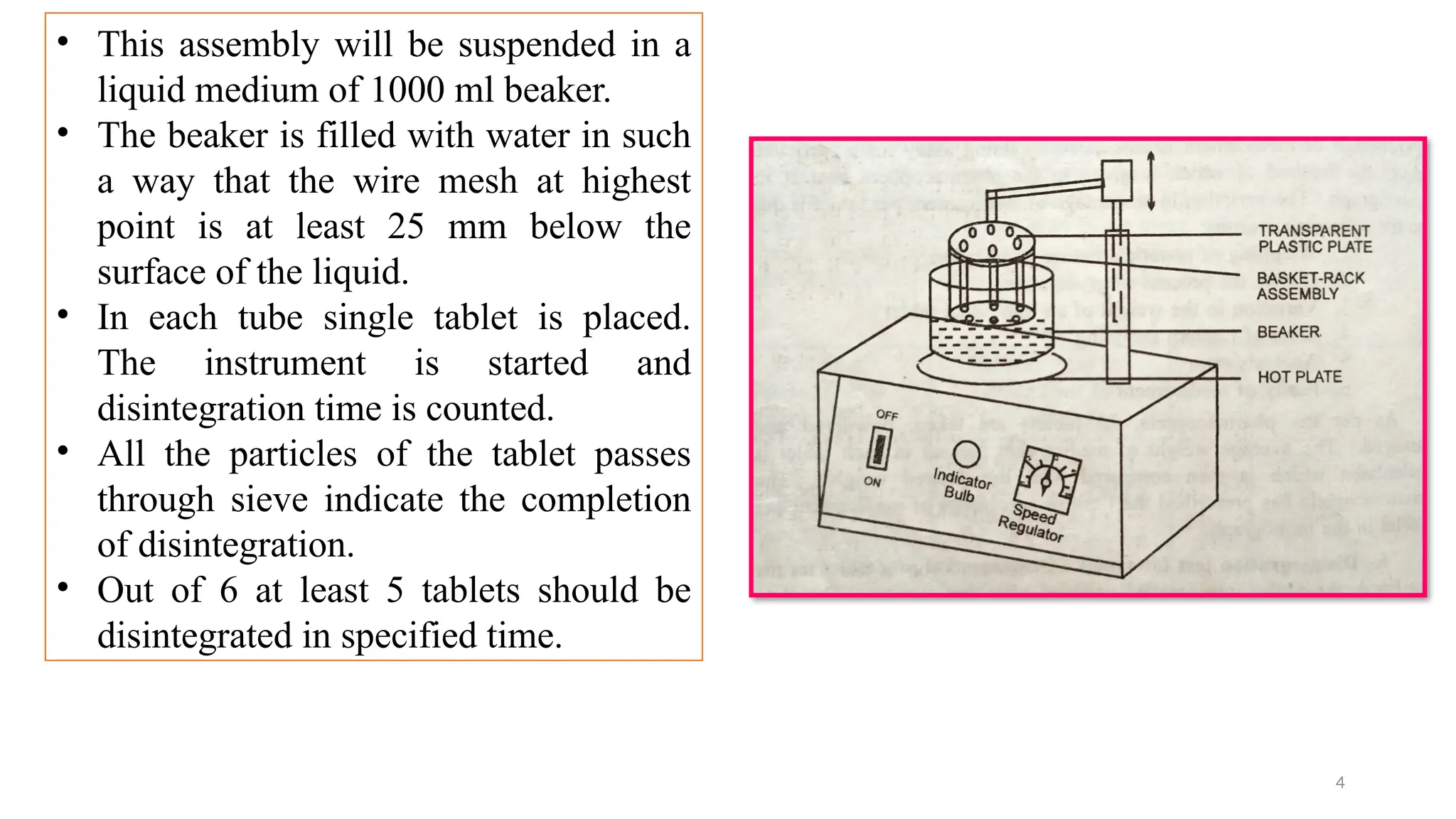
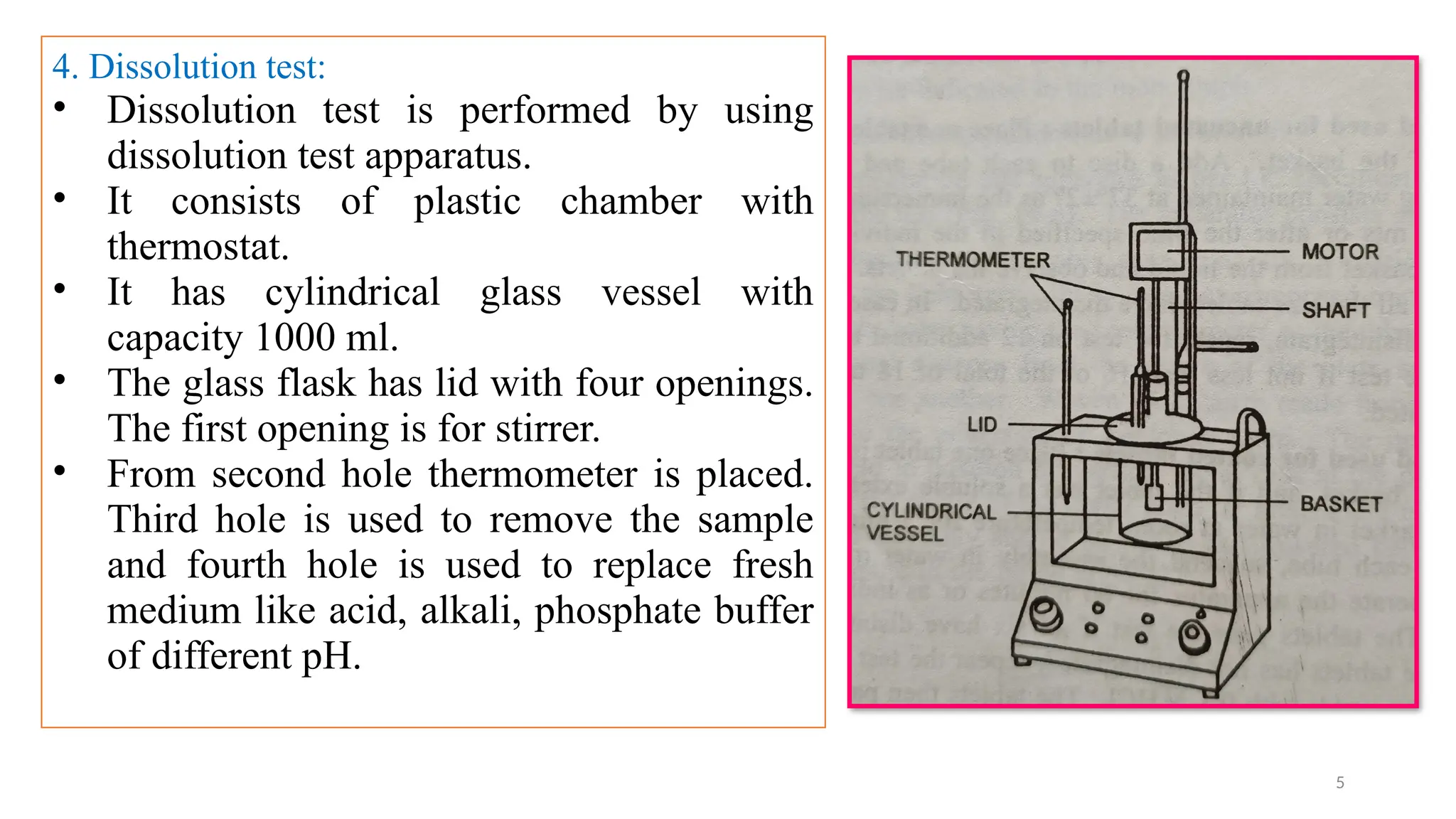
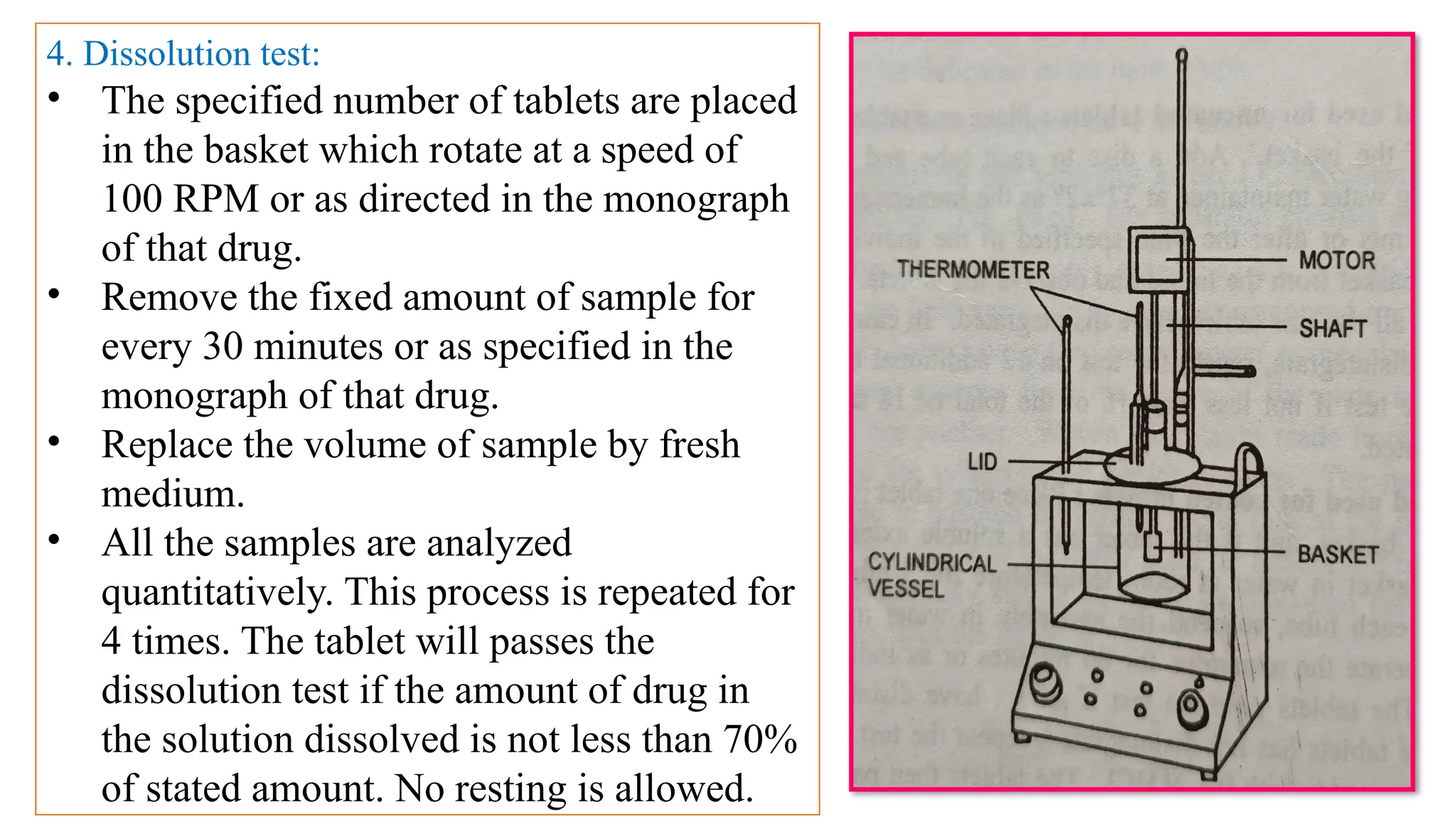
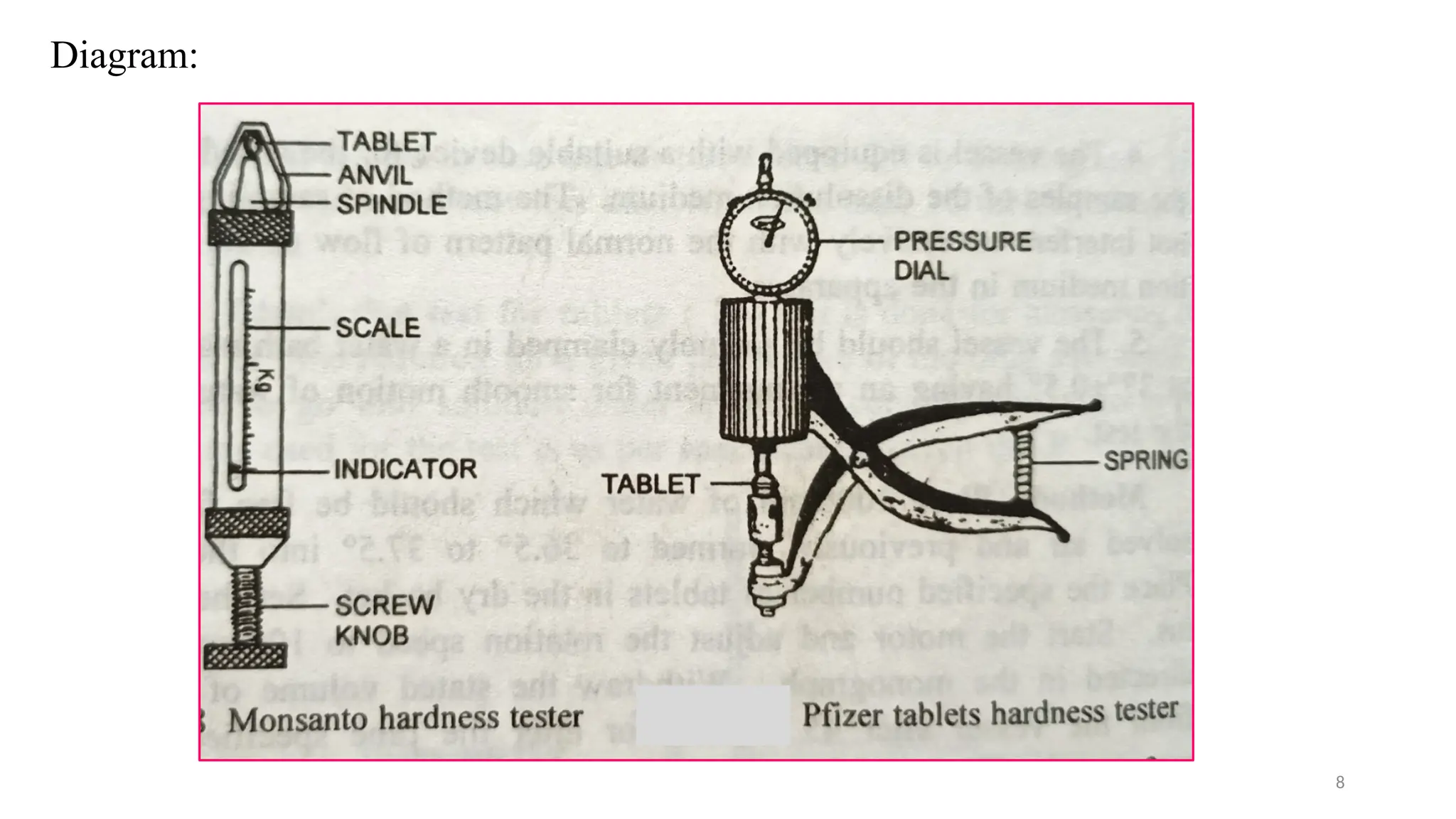
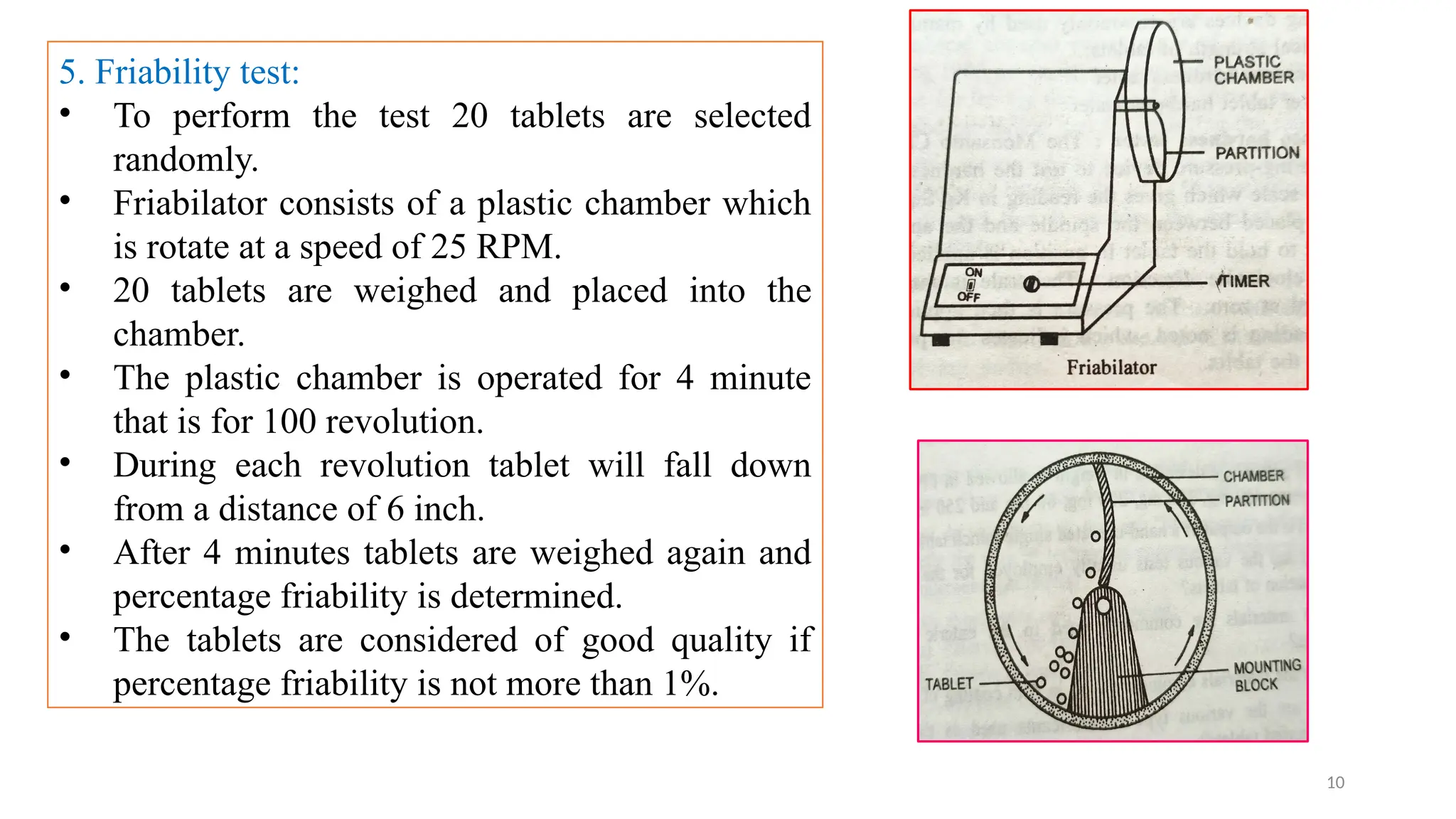
The document outlines both official and unofficial quality control tests for tablets, including content uniformity, weight variation, disintegration, and dissolution tests as well as characteristics like appearance, size, shape, mechanical strength, and friability. Each test includes specific procedures and criteria for compliance, such as using various analytical techniques for content analysis and specific equipment for disintegration and dissolution assessments. Overall, the quality control processes are designed to ensure that tablets meet required specifications for safety and efficacy.