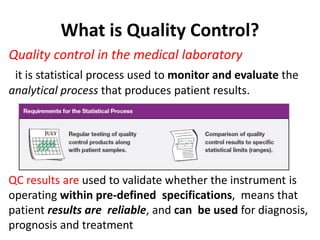
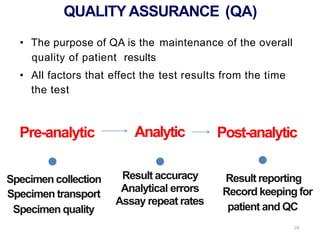
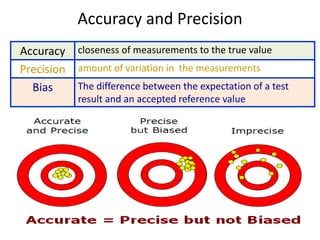
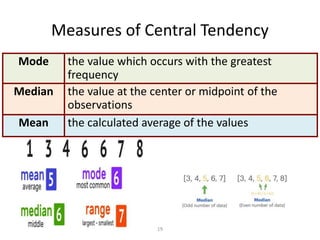
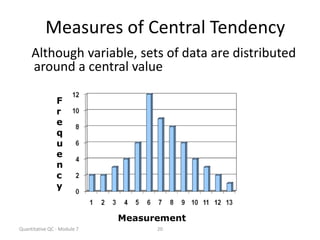
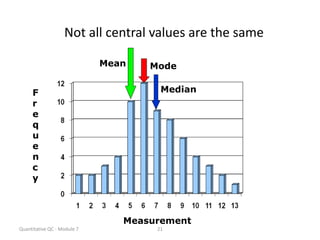
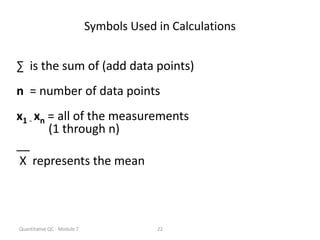
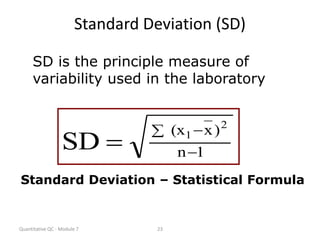
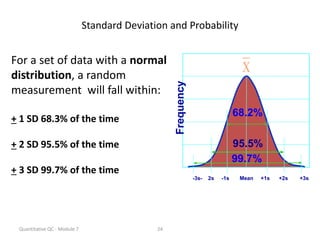
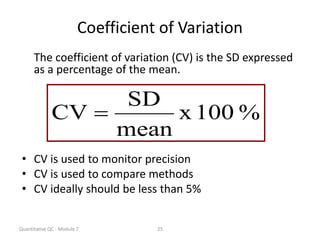
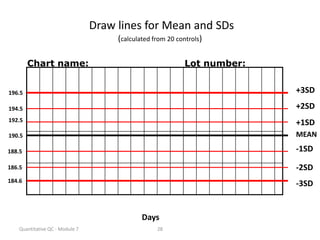
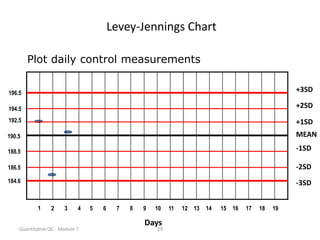
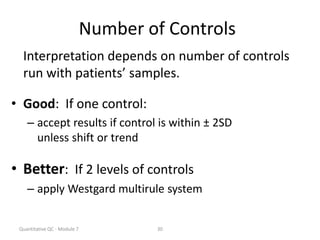
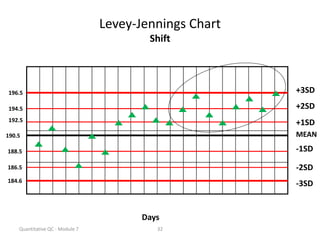
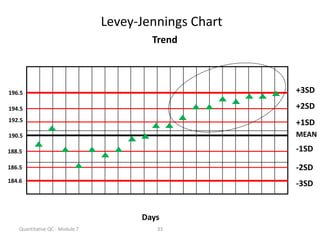
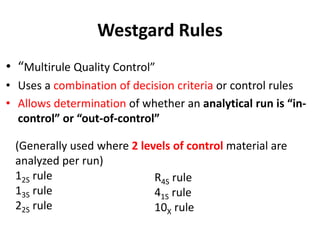
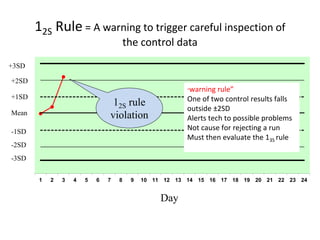
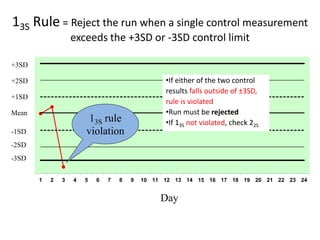
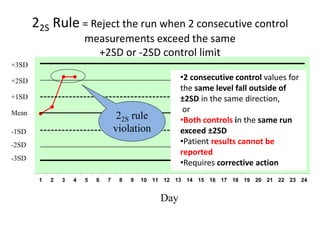
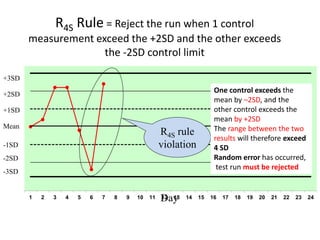
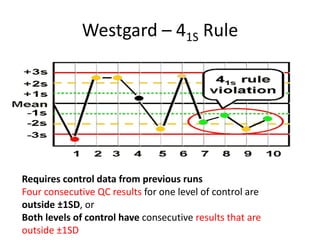
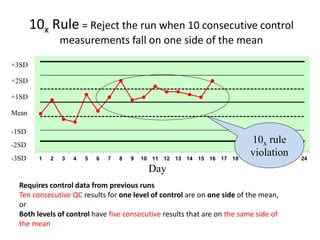
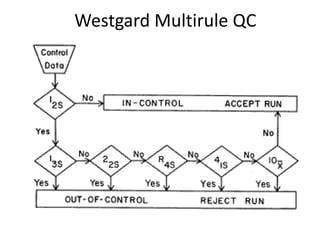
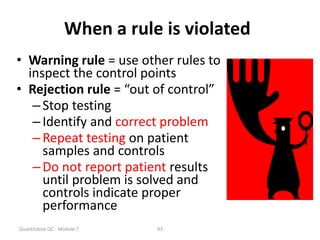
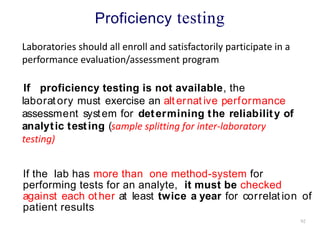
Quality control in medical laboratories involves statistical processes to monitor analytical testing and ensure patient results are reliable for diagnosis and treatment. Control results are used to validate that laboratory instruments are operating within predefined specifications. A quality control program involves establishing written policies and procedures, assigning responsibilities, training staff, obtaining control materials, collecting and analyzing control data, setting target values and control limits, and documenting quality control activities. Control charts are commonly used to compare control results to their known values and identify potential issues requiring corrective action.