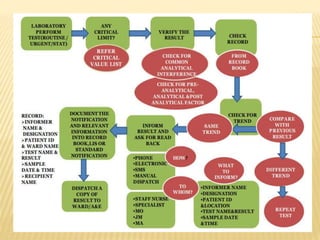
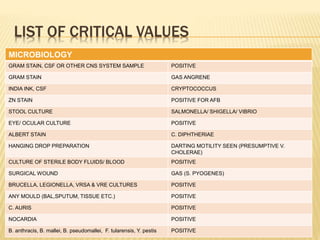
This document defines critical and alert values for microbiology test results. Critical values require immediate notification to clinicians, while alert values require timely intervention. Any positive results for certain dangerous pathogens like Cryptococcus in CSF or AFB in sputum smears are considered critical. Personnel must verify critical results, notify clinicians within 30 minutes, and document all communication. The laboratory has escalation procedures if the responsible clinician cannot be contacted. Proper patient identification and result verification steps must be followed to privately notify the healthcare team by phone. Various microbiology test results that would be reported as critical values are also listed.