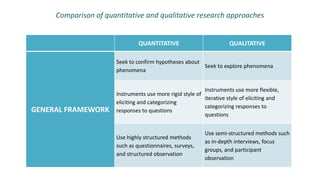
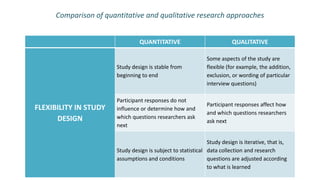
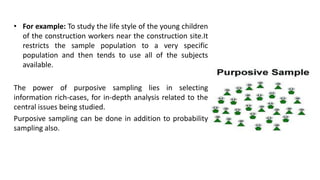
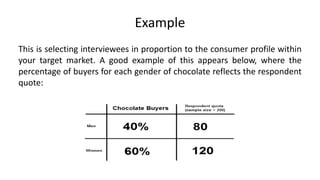
Here are the key points about informed consent:
- It is a process, not just a form. Researchers must ensure participants understand what participation involves through clear verbal and written explanations.
- Consent forms should be written in plain, easy-to-understand language appropriate for the population.
- Participants must be able to refuse or withdraw from the study without penalty.
- Risks and limitations of confidentiality should be clearly explained.
- Participants should have the opportunity to ask questions to fully comprehend what they are consenting to.
- Informed consent is an ongoing process, not a single event, with the option for participants to withdraw later.
The goal is to respect participants' autonomy by