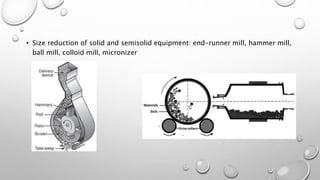
This document provides an overview of semisolid preparations for an assignment. It defines semisolid preparations and their ideal properties. It describes the advantages and disadvantages of semisolid dosage forms. It also outlines the main types of semisolid preparations, common ingredients used, evaluation methods, and equipment involved in formulation. Key points covered include the properties of bases, role of antioxidants and humectants, methods for testing drug content uniformity and spreadability, and importance of temperature control during processing.