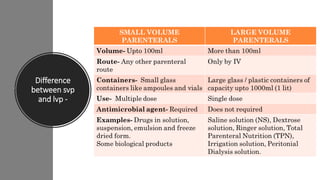
The document provides an overview of parenteral dosage forms, which are sterile drug products administered through injection. It categorizes them into large volume parenterals (LVP) and small volume parenterals (SVP), detailing their uses, characteristics, and formulation considerations. Ideal parenteral products are sterile, isotonic, and free from pyrogens, with specific preparation guidelines to ensure safety and efficacy.