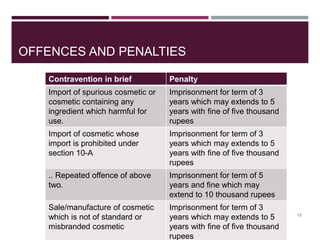
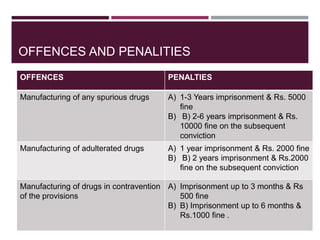
The document outlines the regulatory framework governing cosmetics in India under the Drug and Cosmetic Act of 1940, detailing definitions, import prohibitions, labeling requirements, and manufacturing guidelines. It highlights the penalties for offenses related to spurious and misbranded cosmetics, emphasizing the need for registration and quality standards as dictated by the Bureau of Indian Standards. Additionally, it covers the requirements for factory premises and equipment used in the manufacturing of cosmetics.