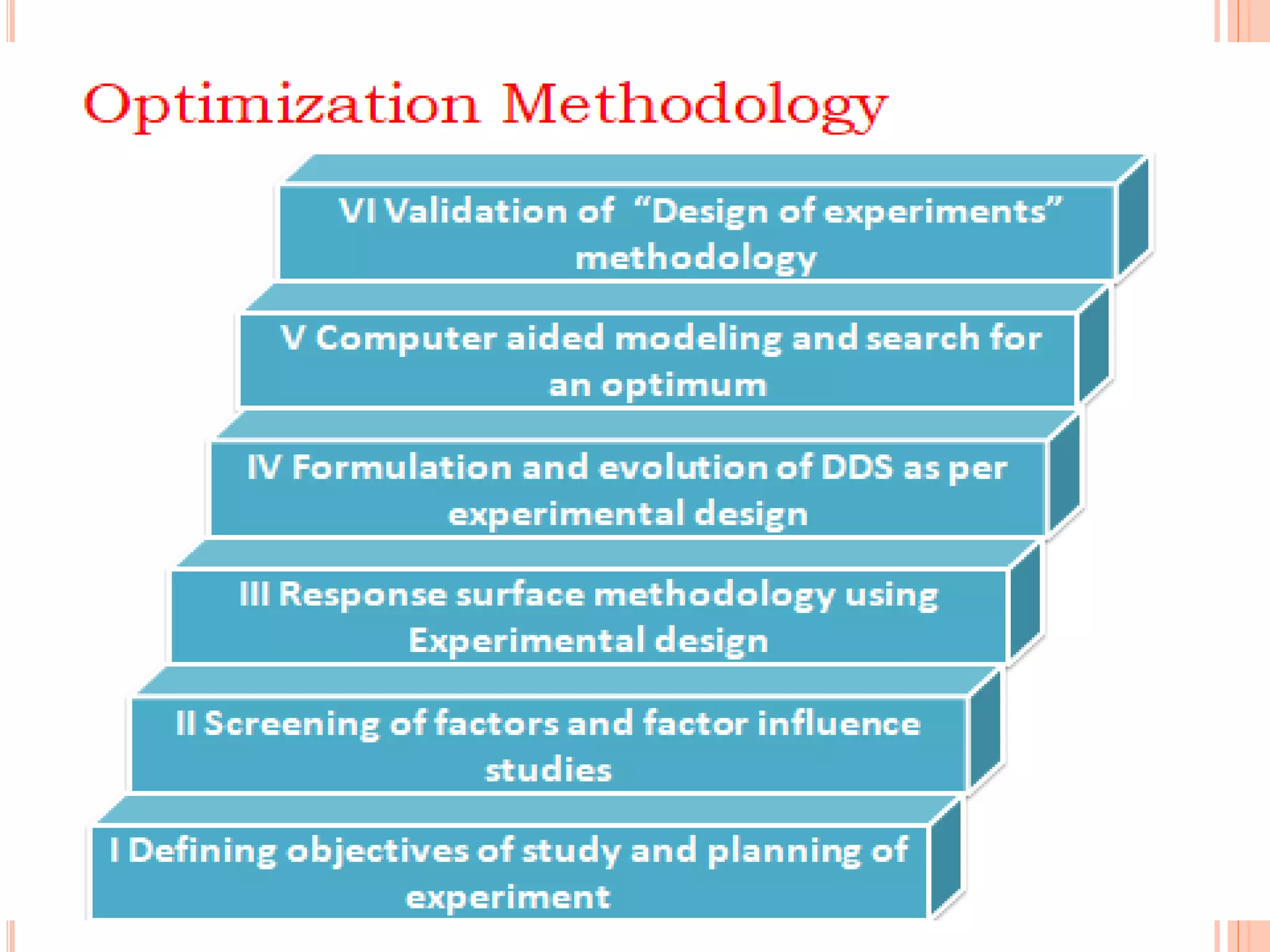
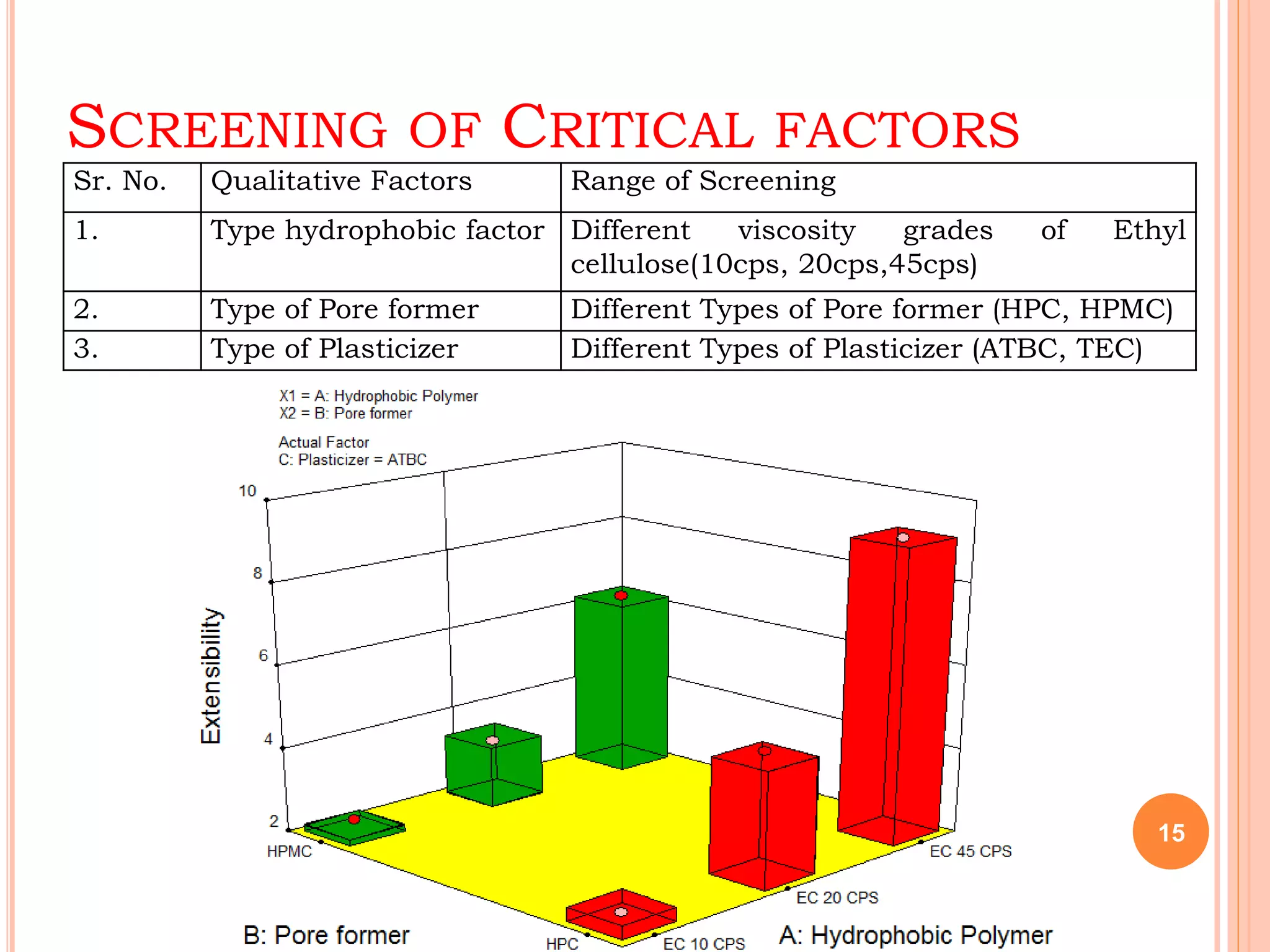
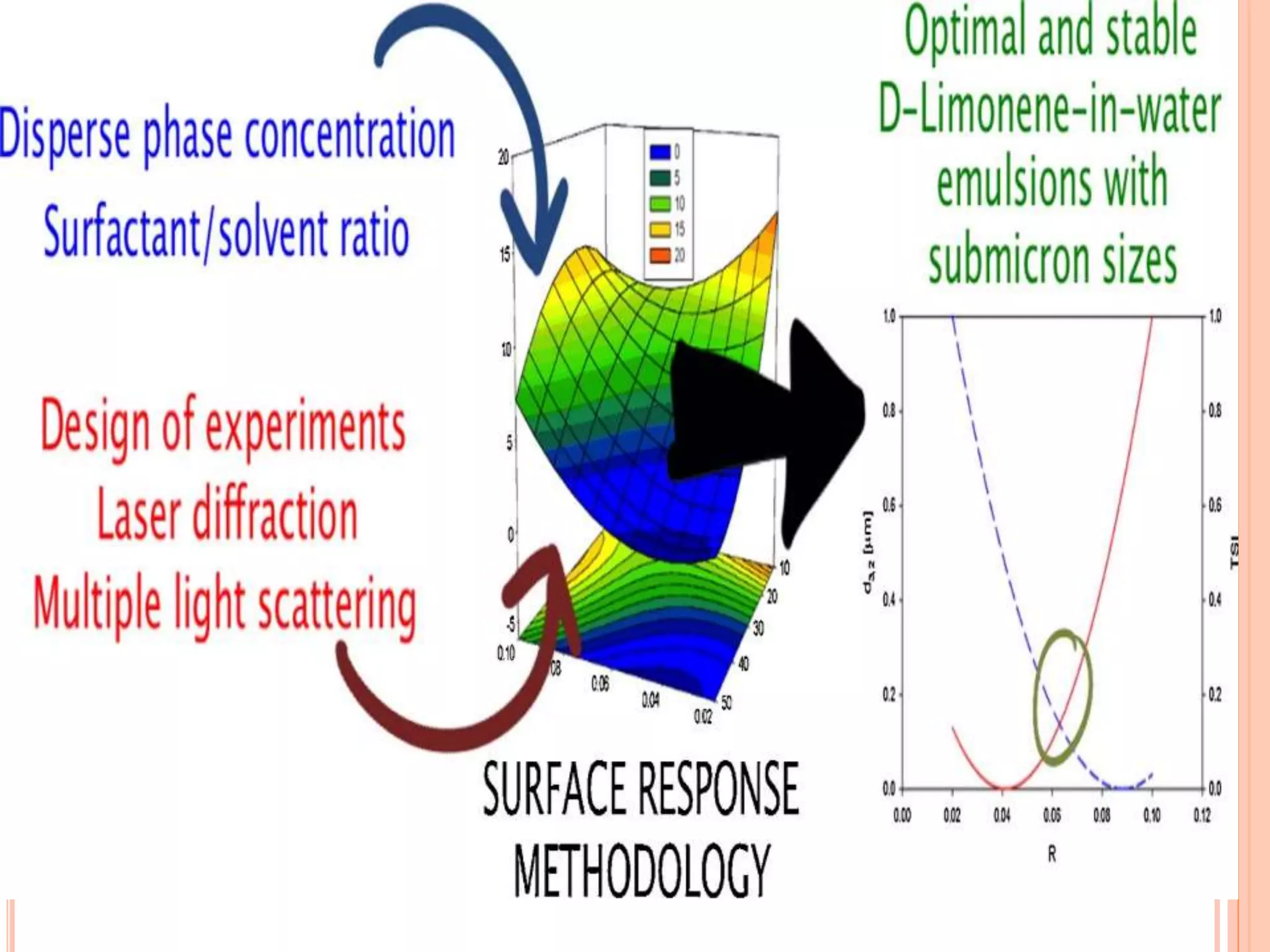
This document discusses the use of computers in pharmaceutical formulation. It begins with an introduction to pharmaceutical formulation and design of experiment techniques. It then provides examples of emulsion and microemulsion formulations. The document reviews several software programs used for design of experiment and optimization in formulation development. It also discusses using design of experiment techniques for screening critical factors and developing different dosage forms. Finally, it covers legal protection of innovative computer uses in research and development, including patents, copyright, database protection, and trade secrets.