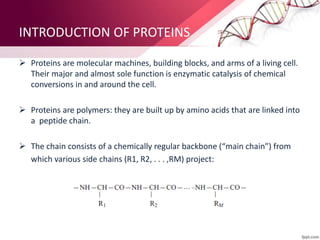
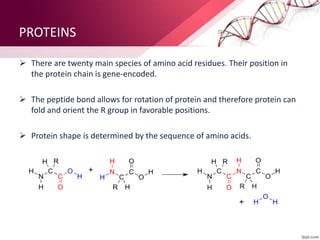
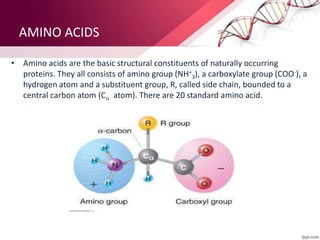
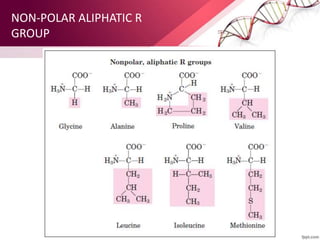
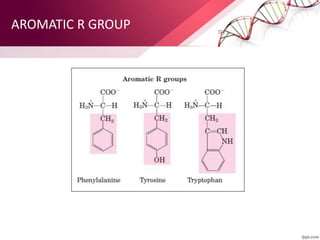
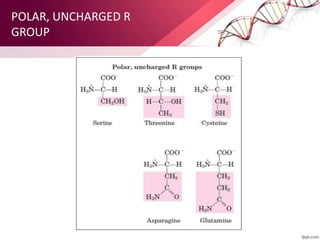
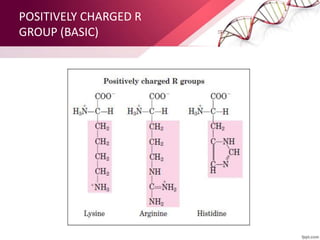
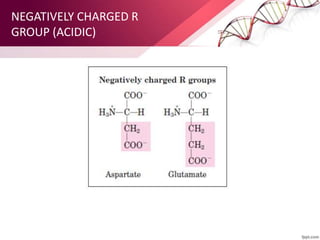
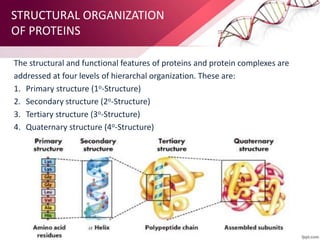
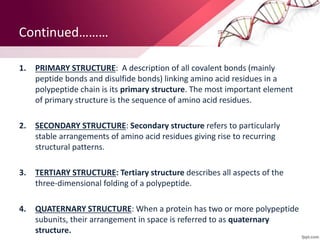
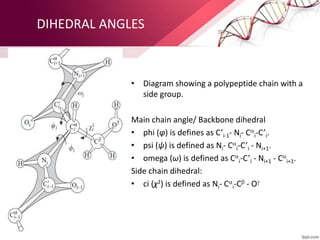
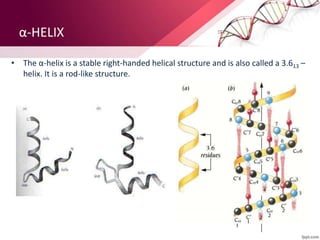
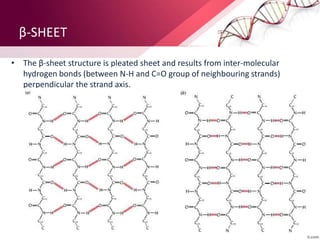
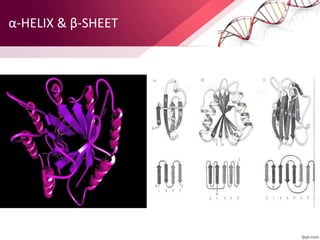
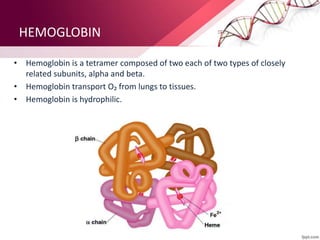
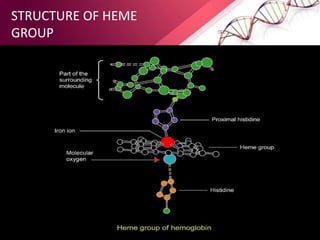
The document provides an overview of proteins, detailing their roles as enzymatic catalysts and their structural organization based on amino acids. It classifies proteins into fibrous, membrane, and globular types and discusses the primary to quaternary structures of proteins, alongside dihedral angles and the structures of hemoglobin and myoglobin. Additionally, it highlights the classification of amino acids based on their side chains into five categories.