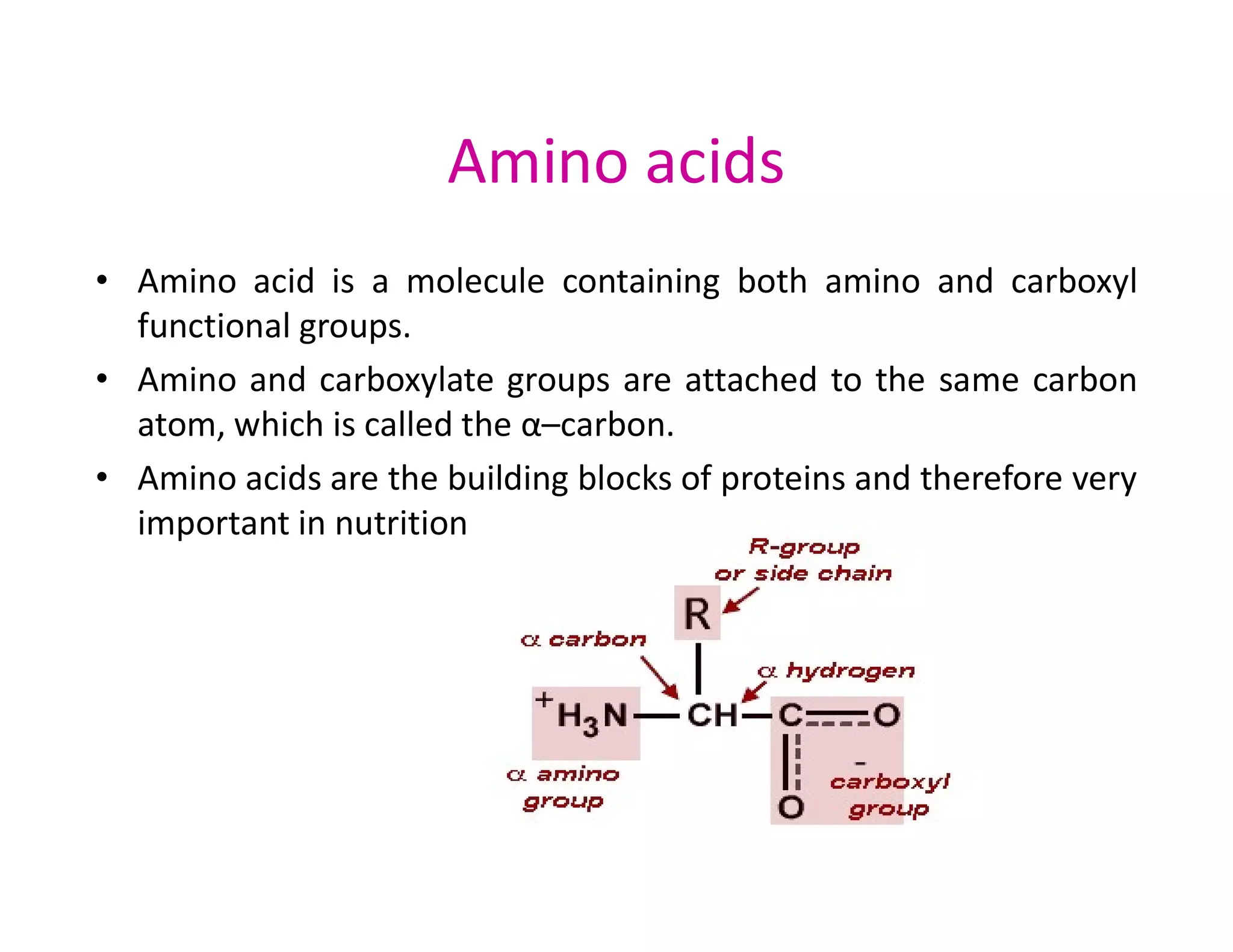
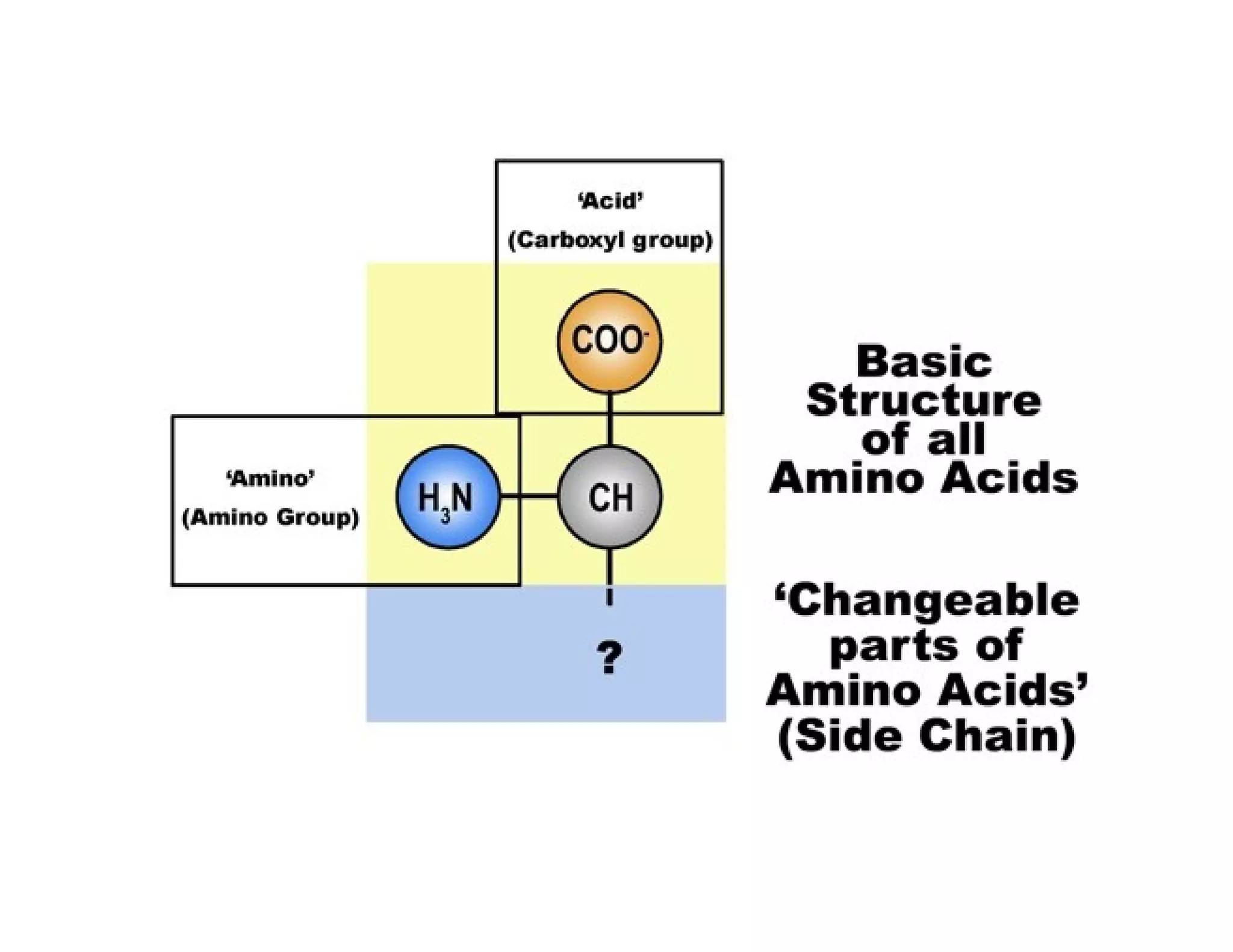
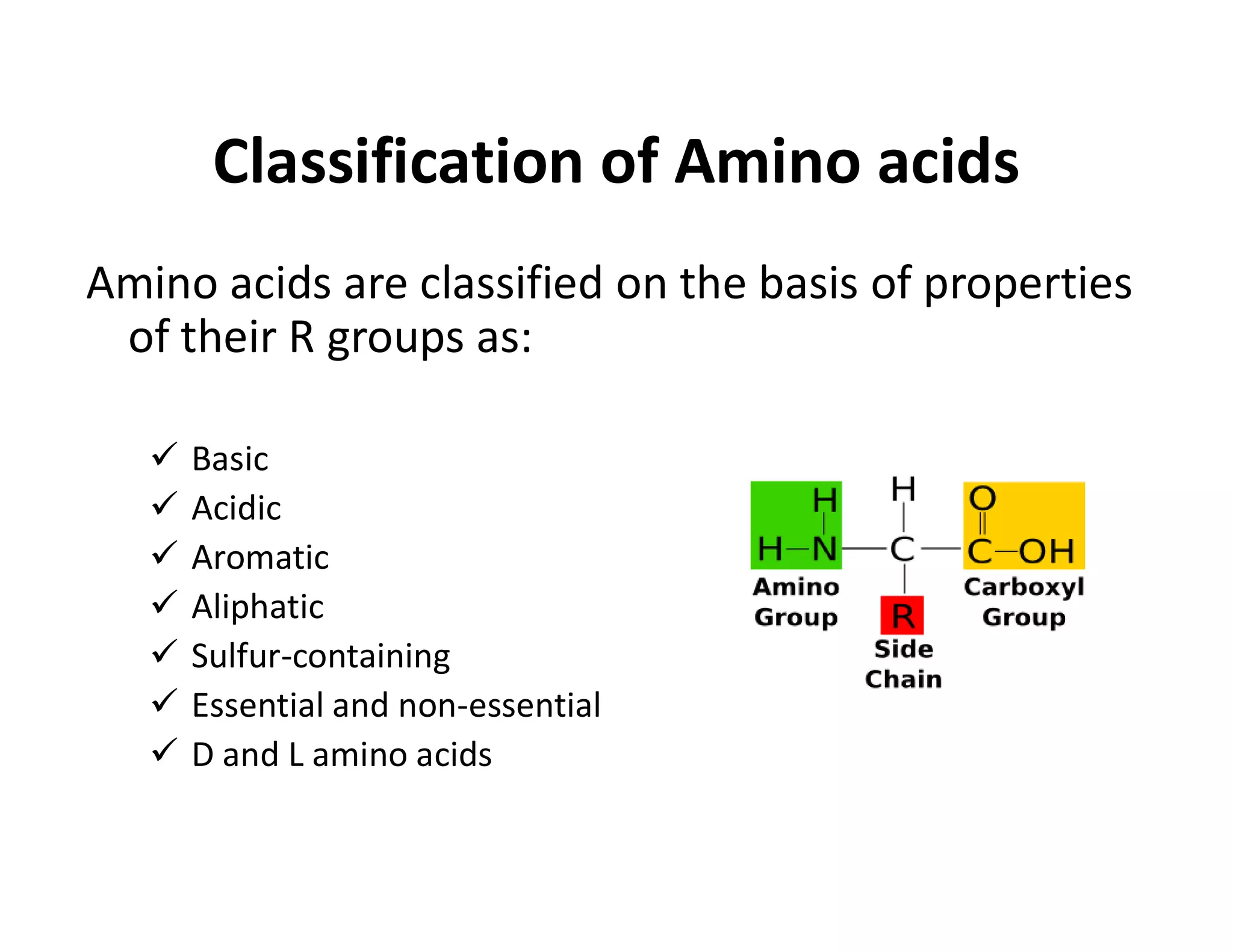
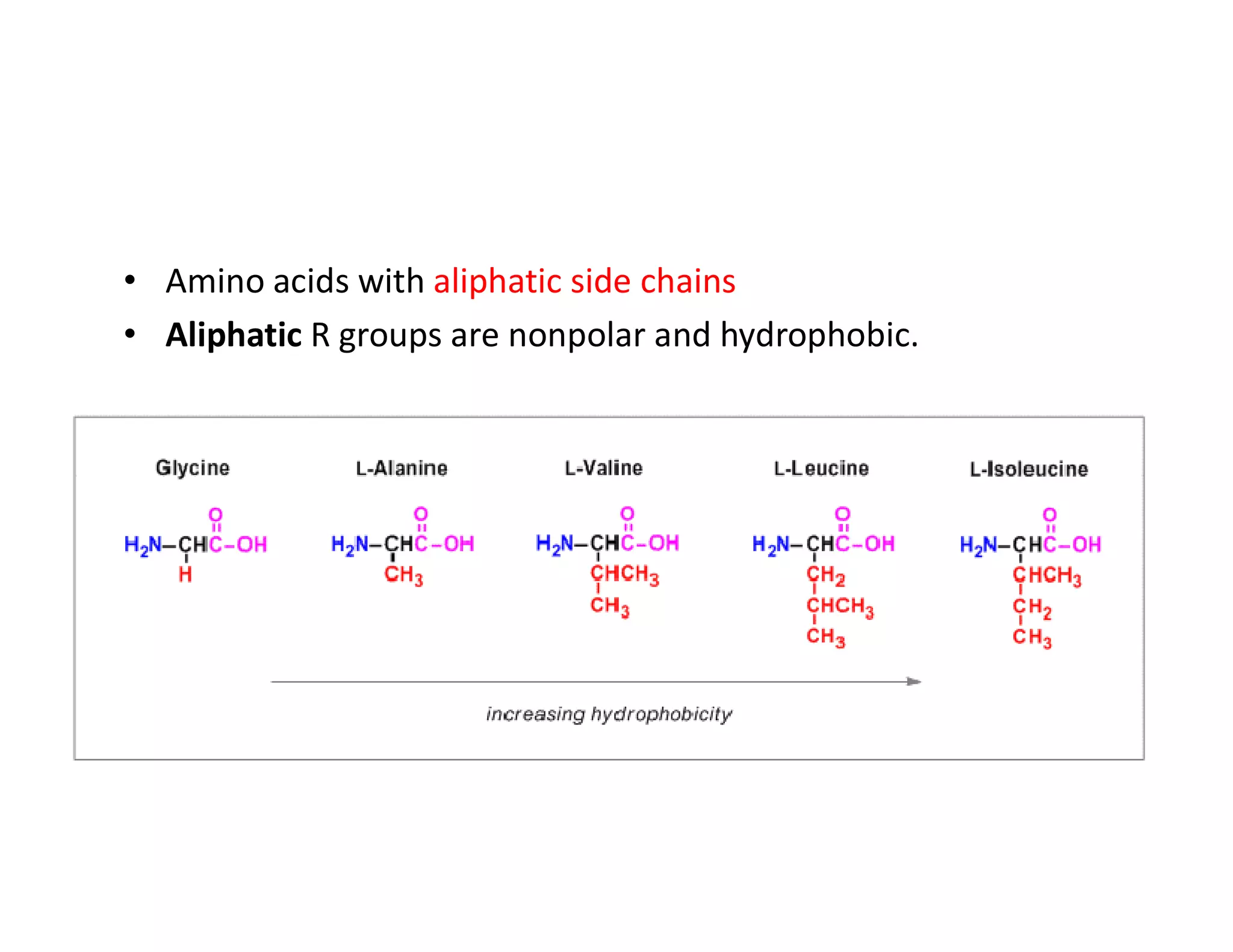
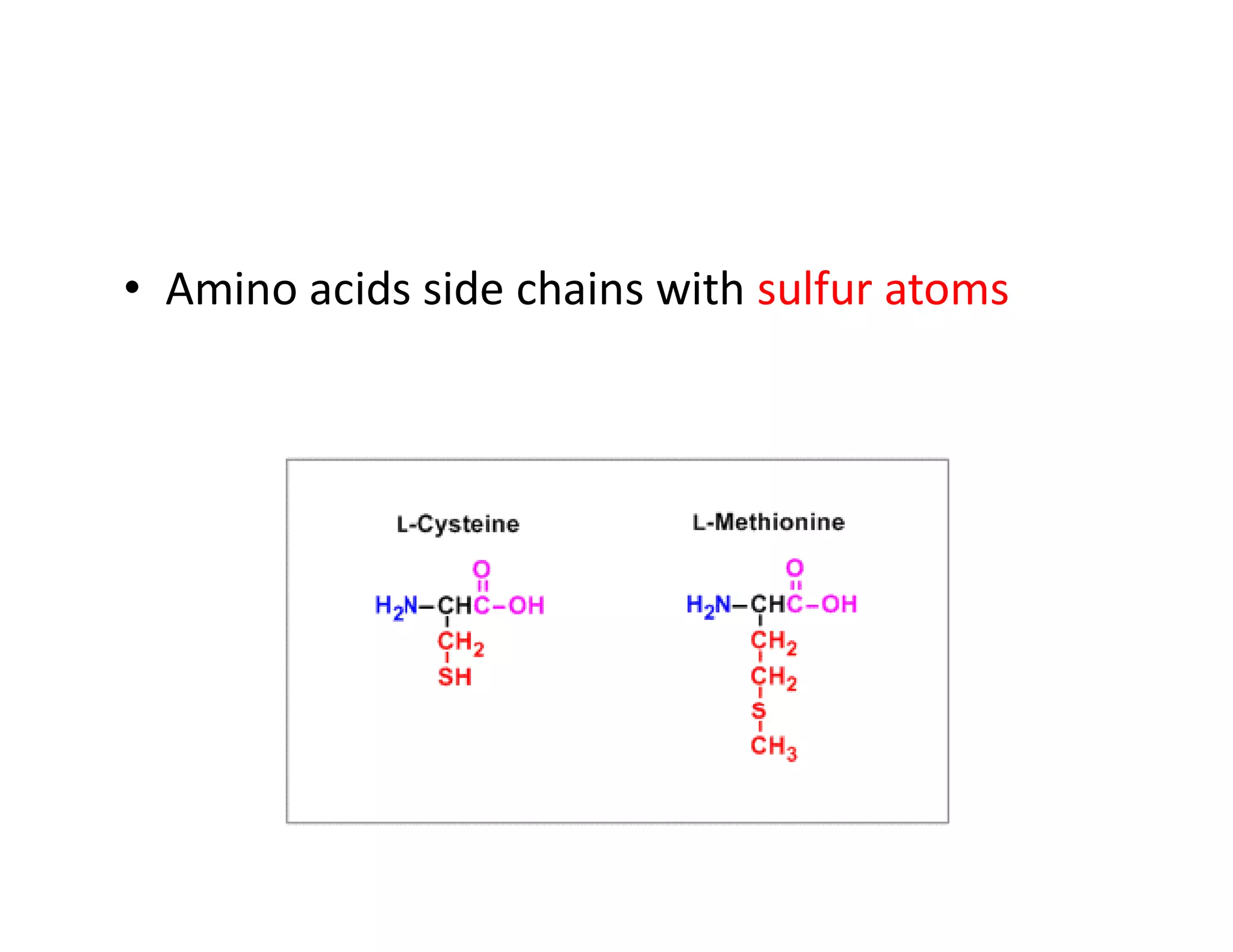
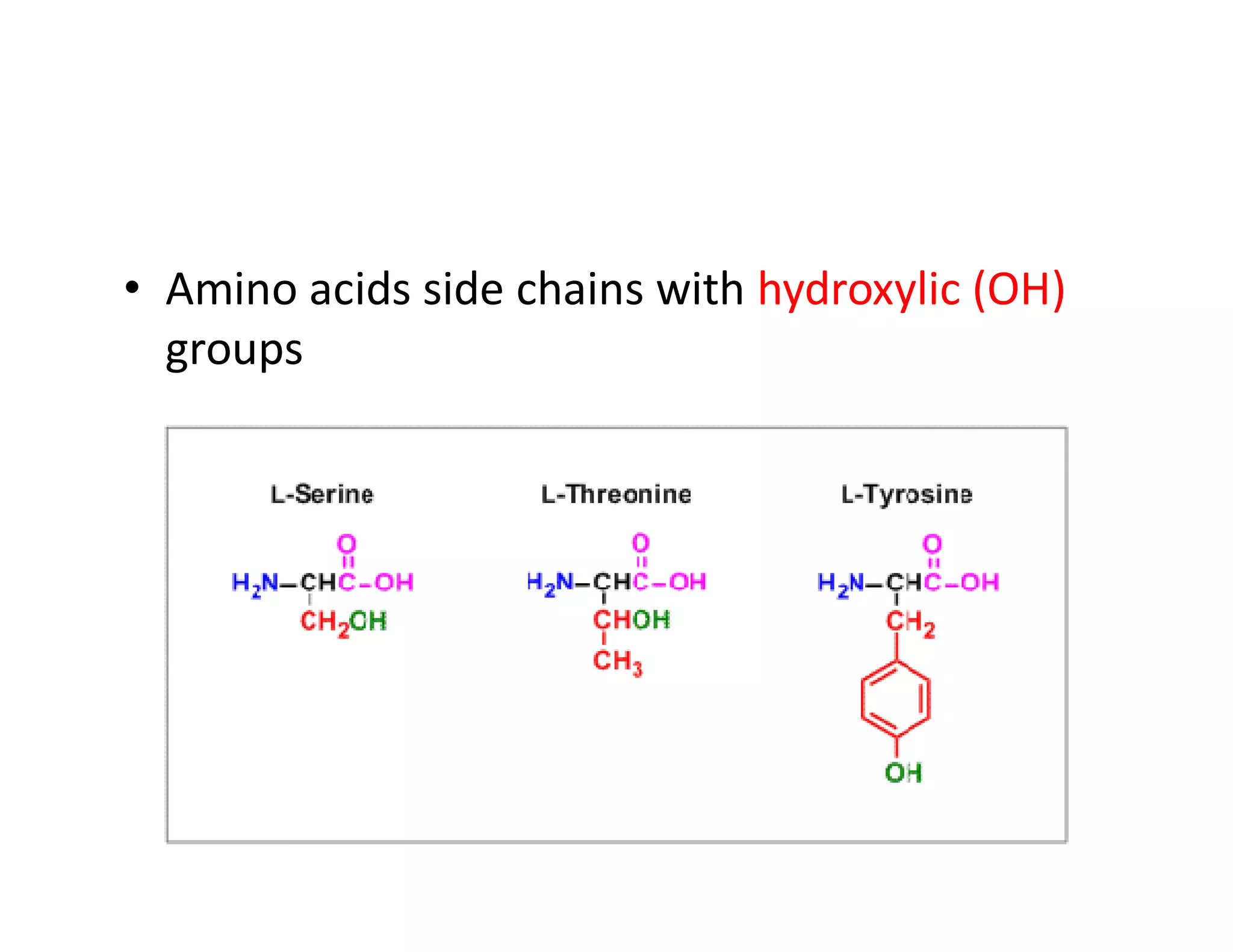
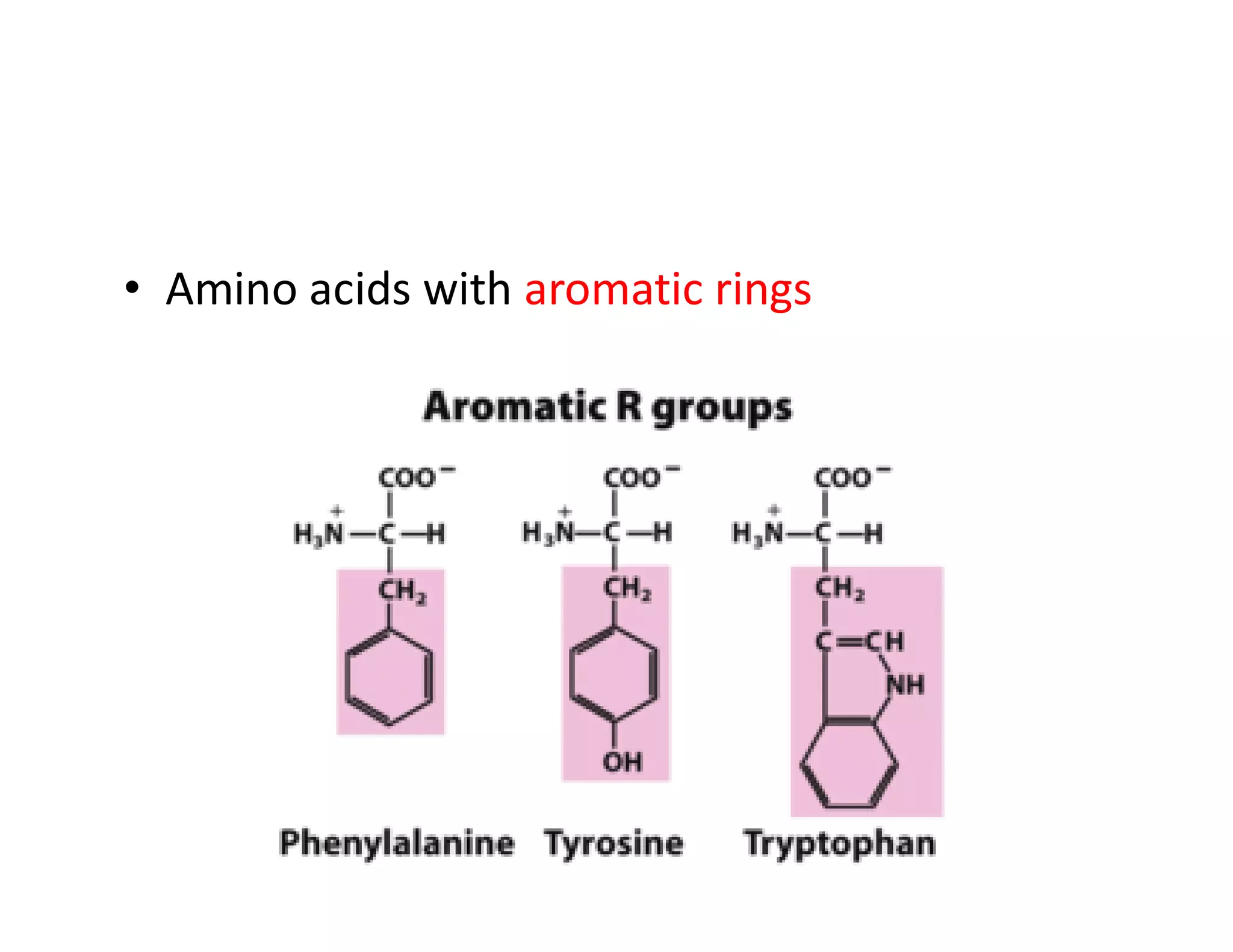
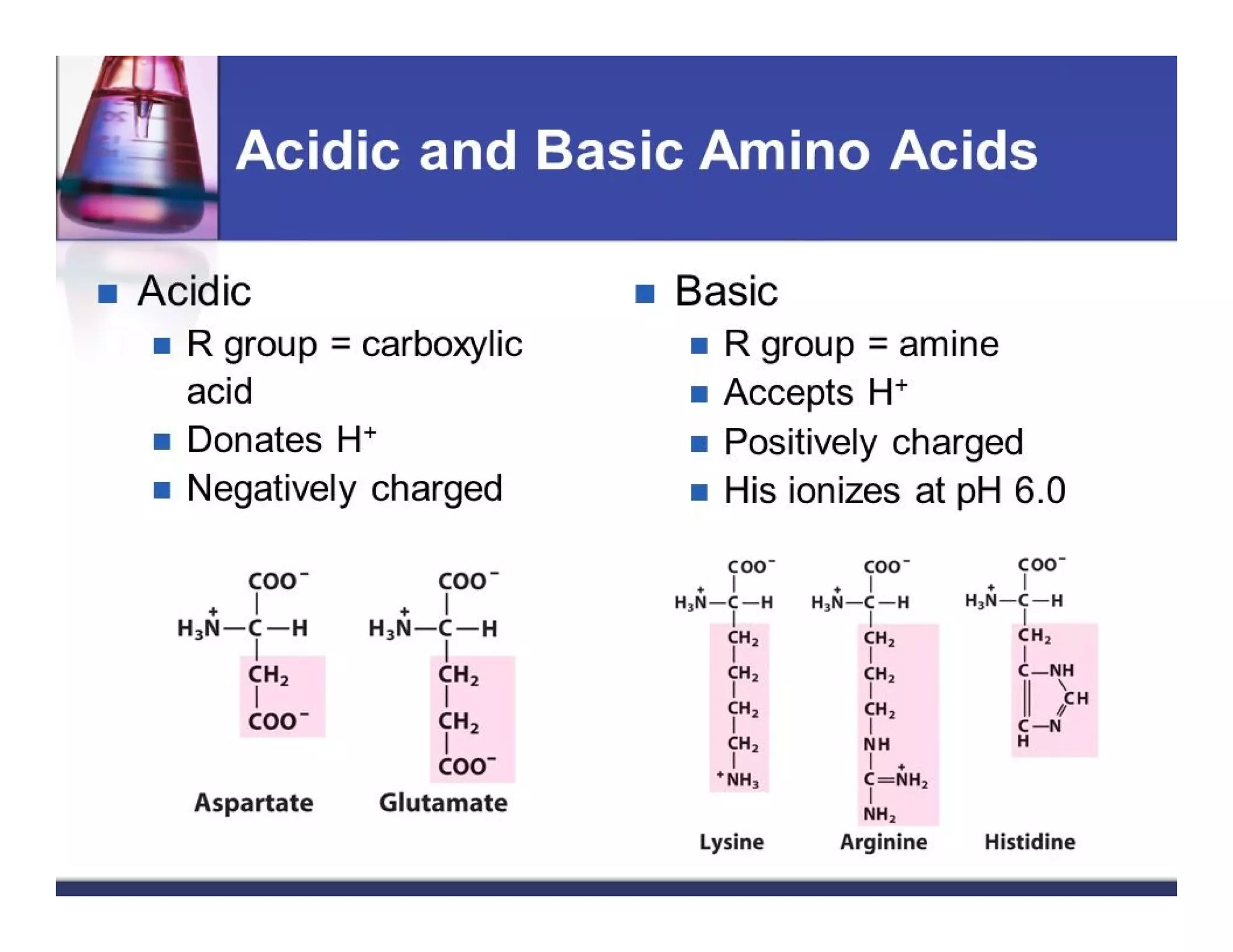
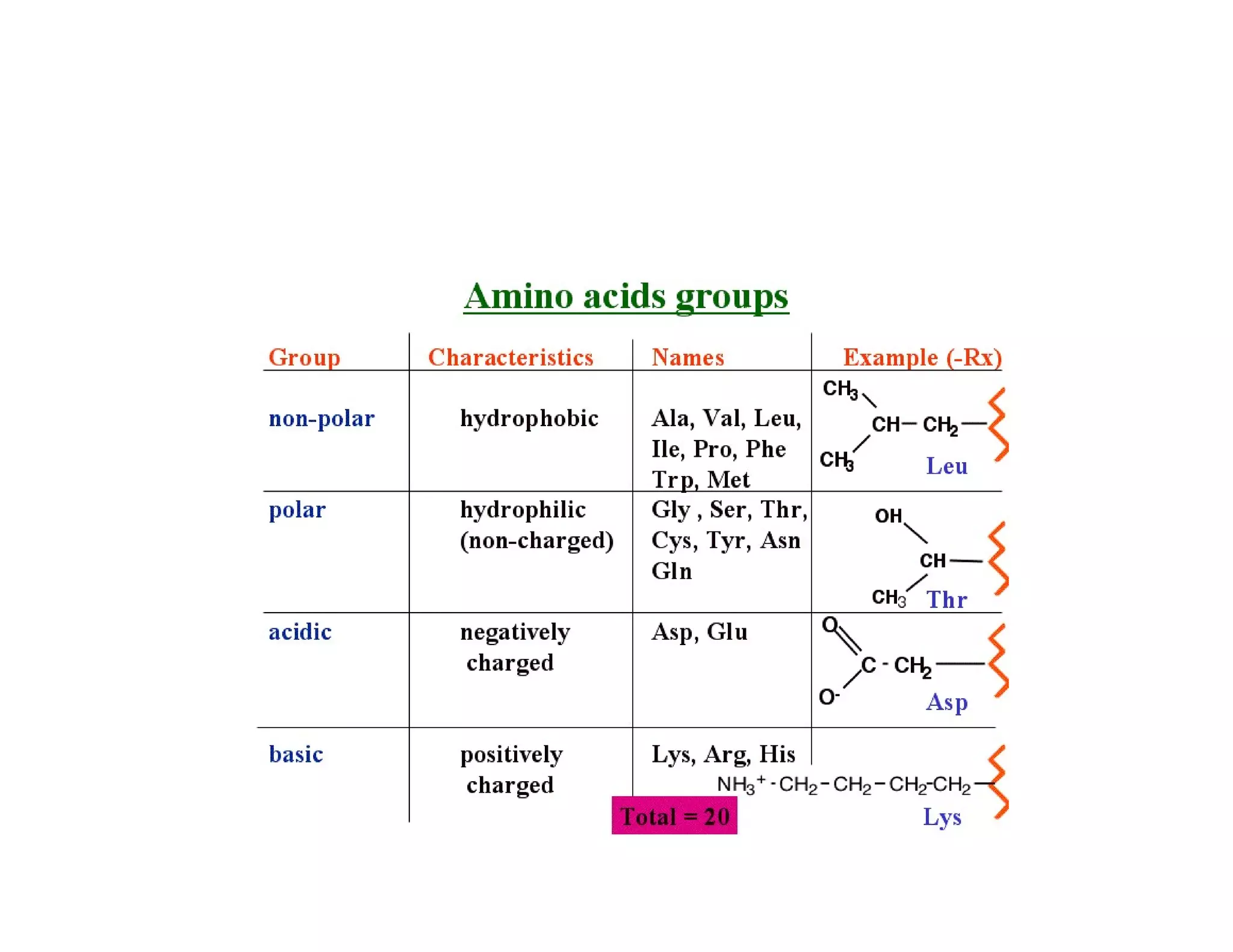
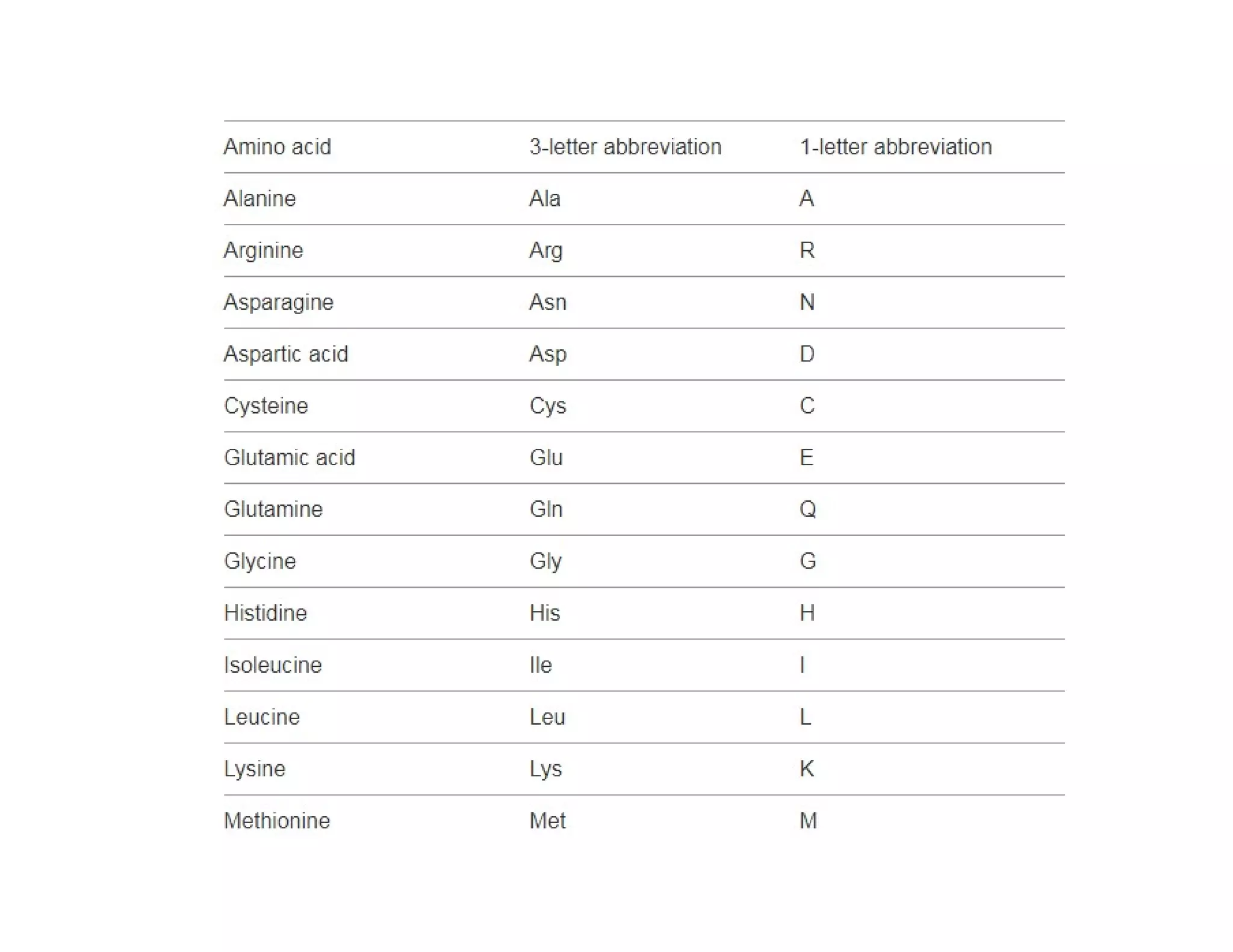
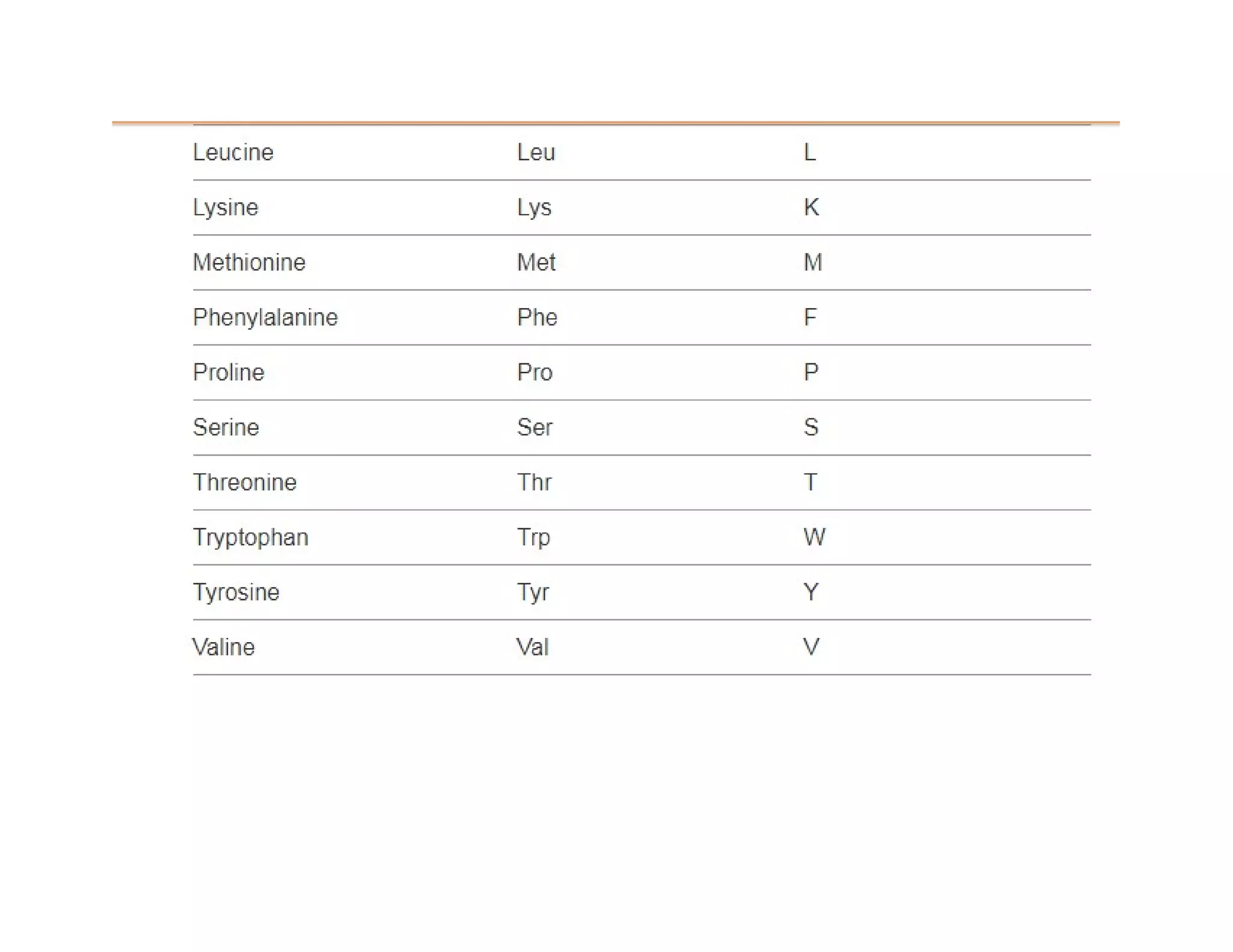
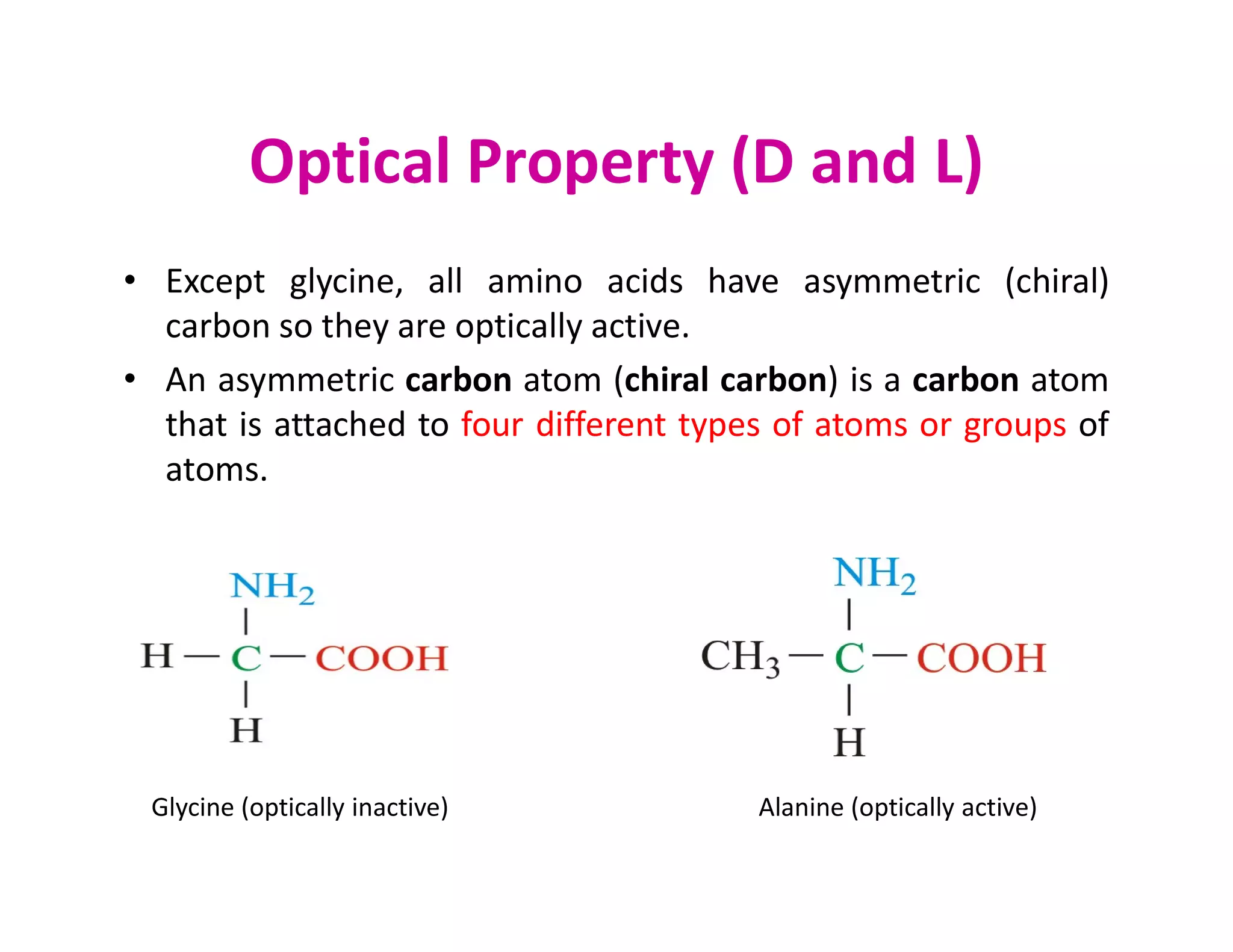
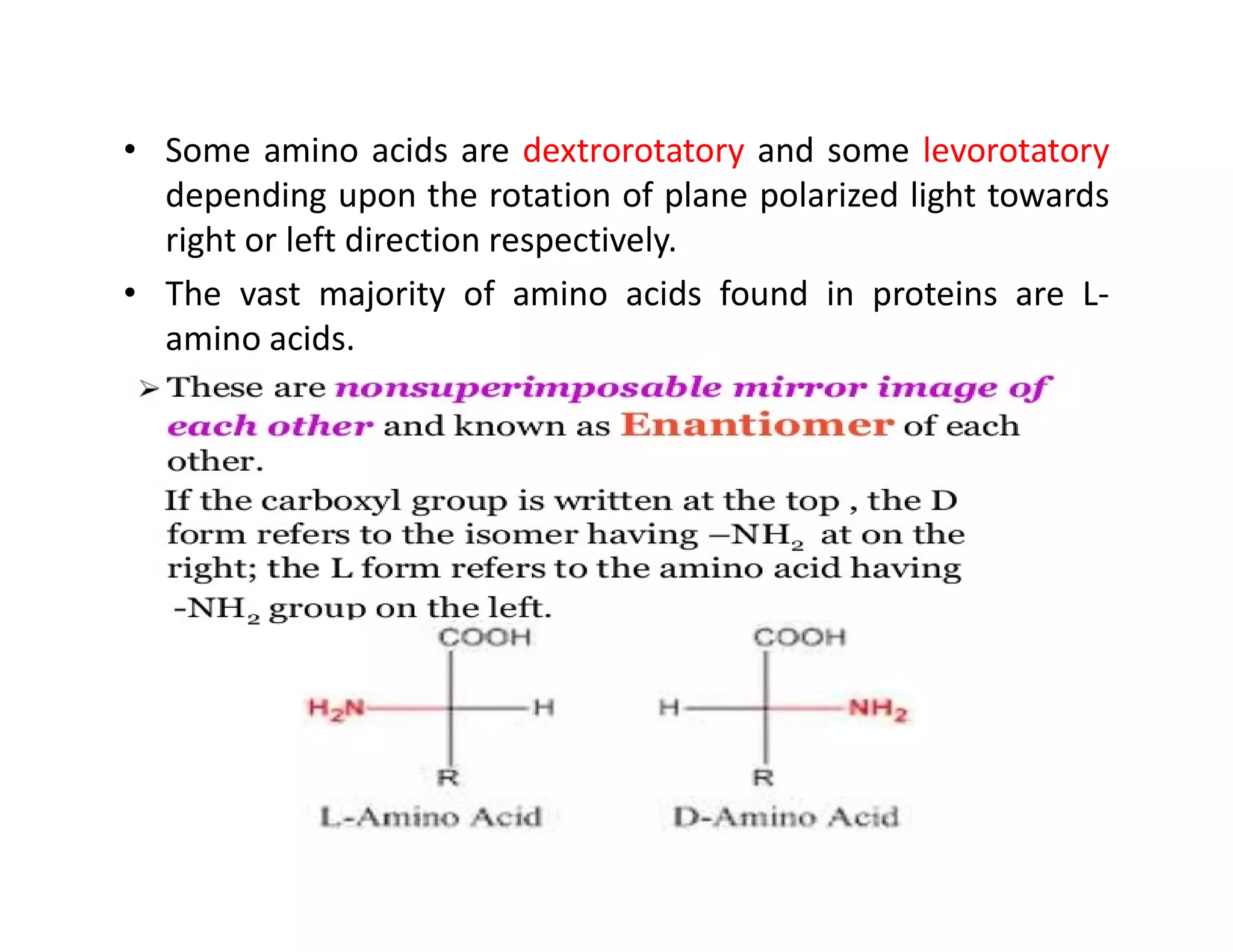
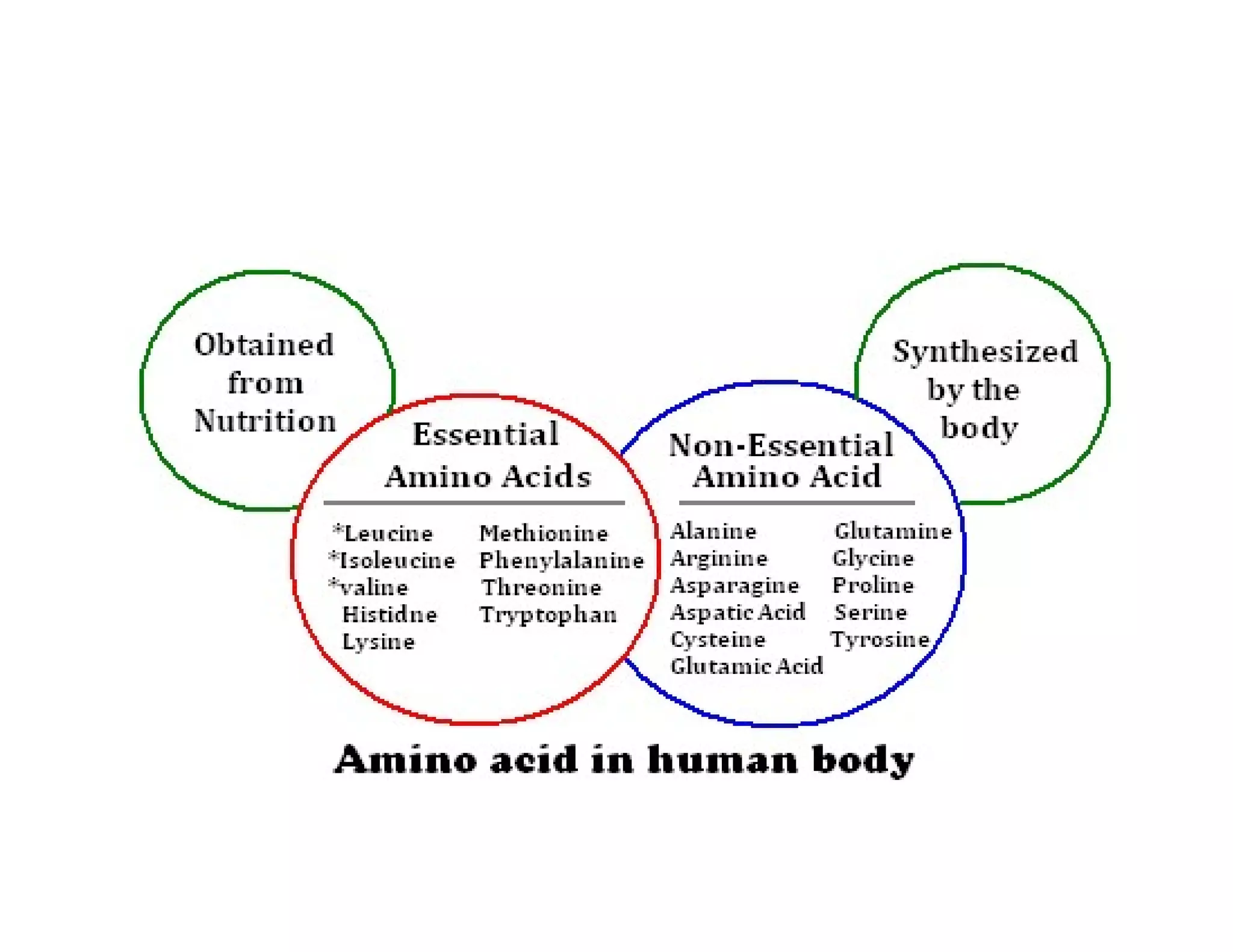
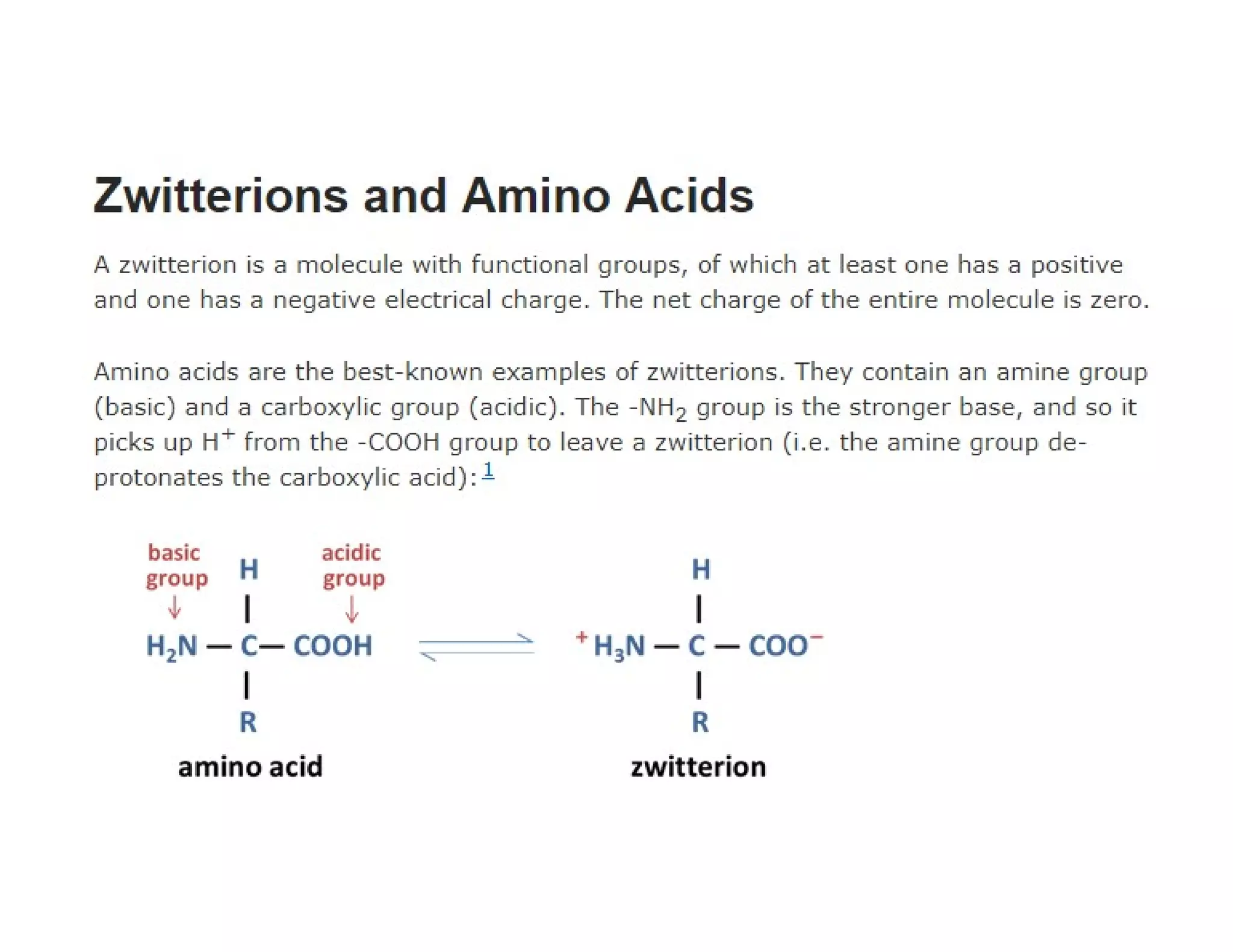
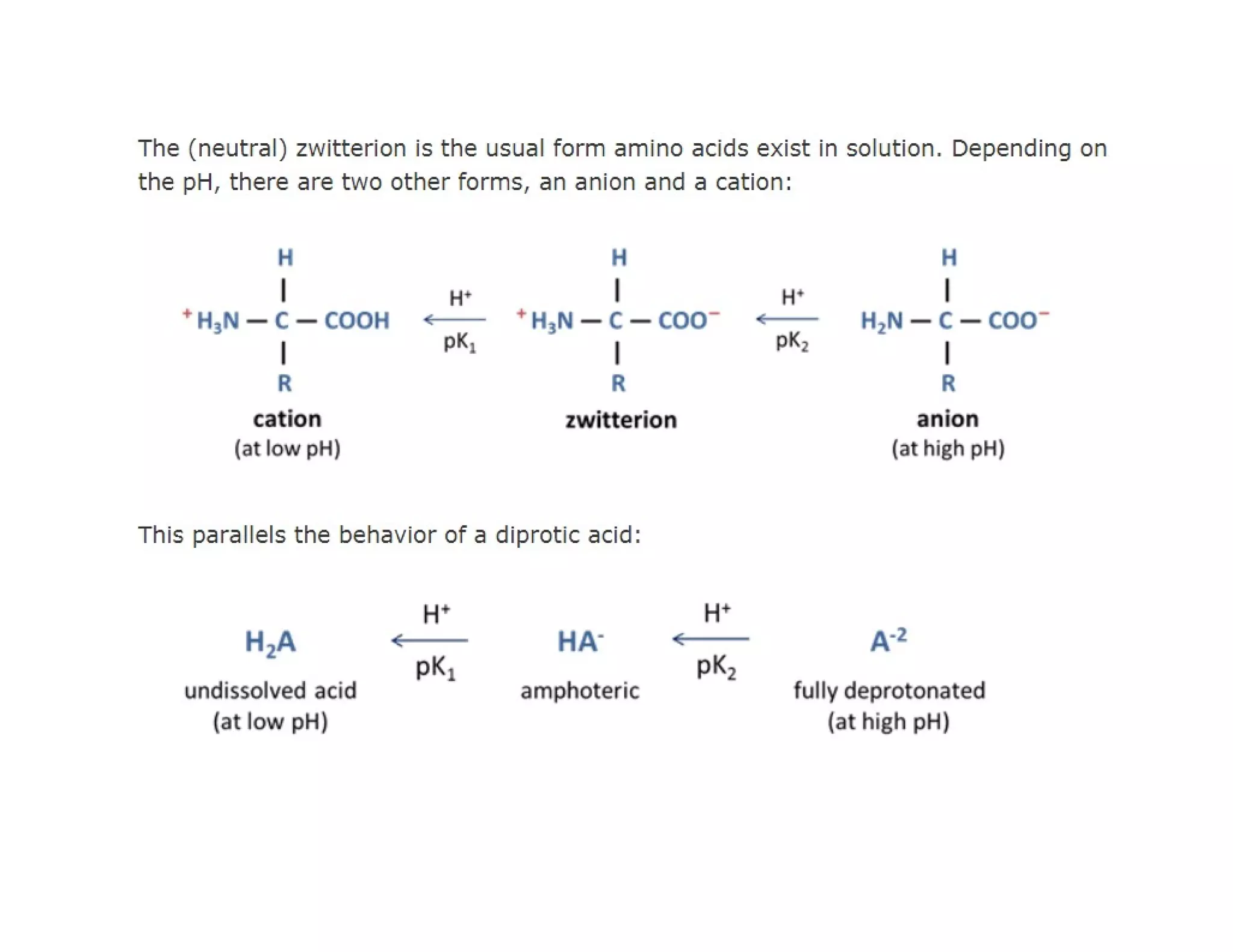
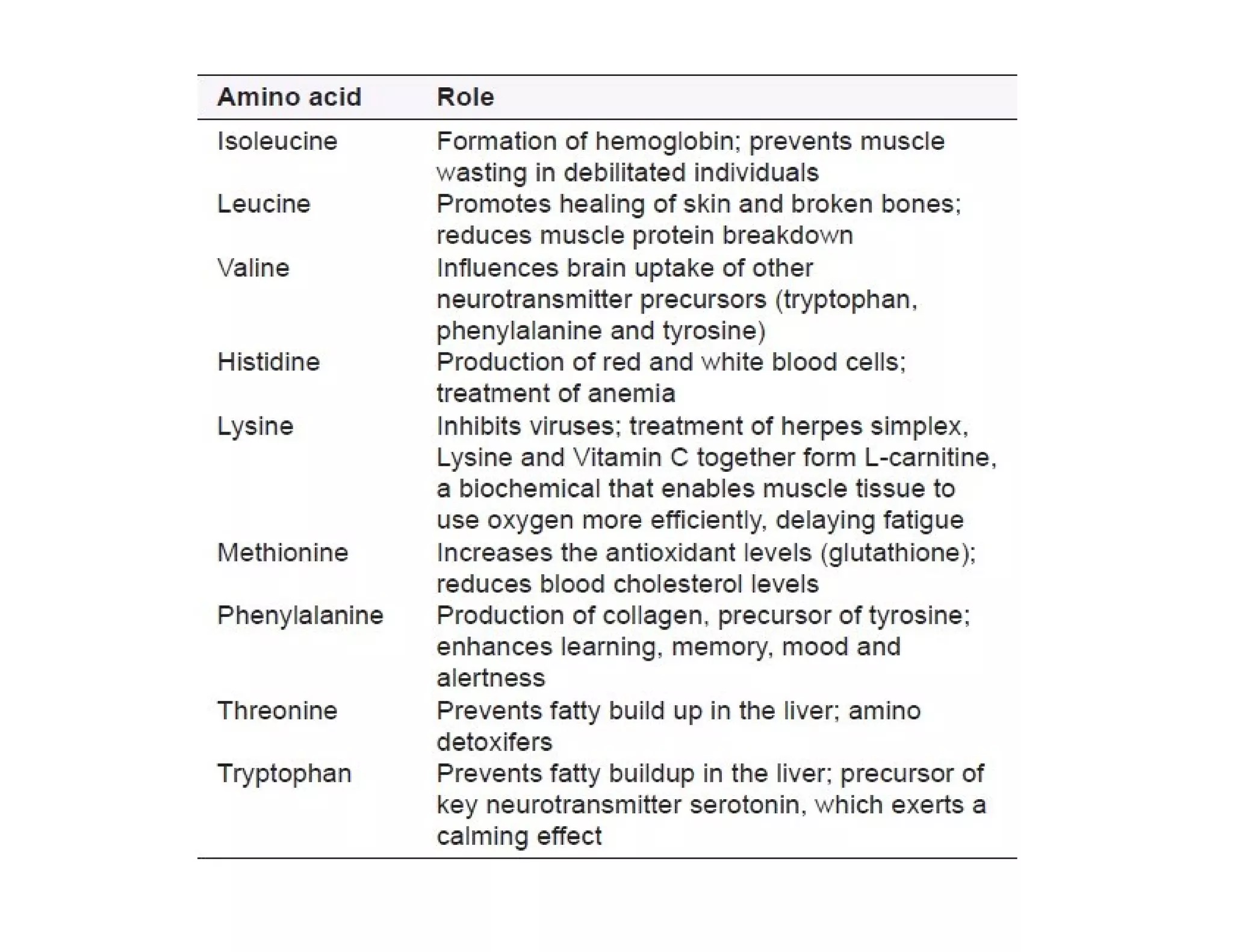
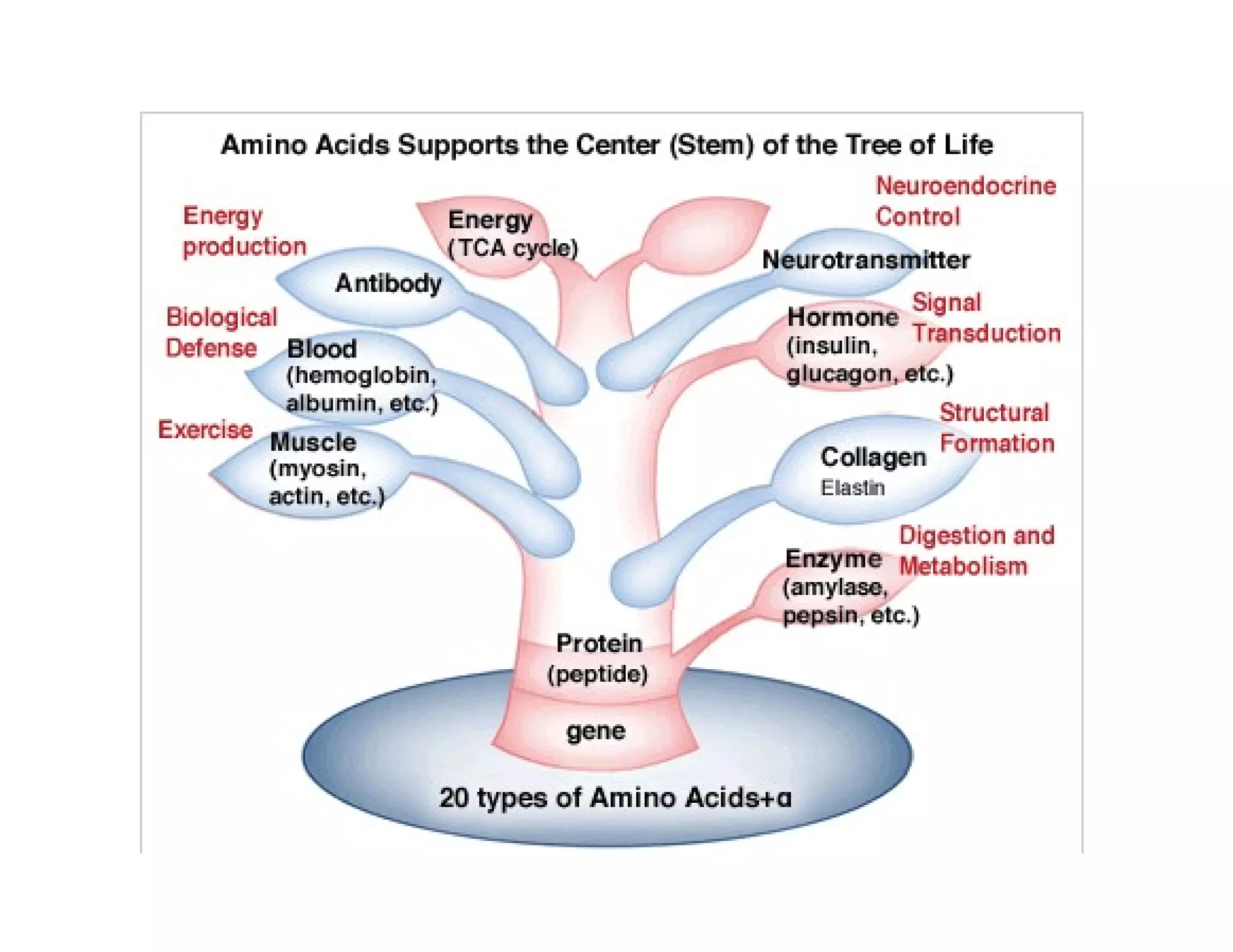
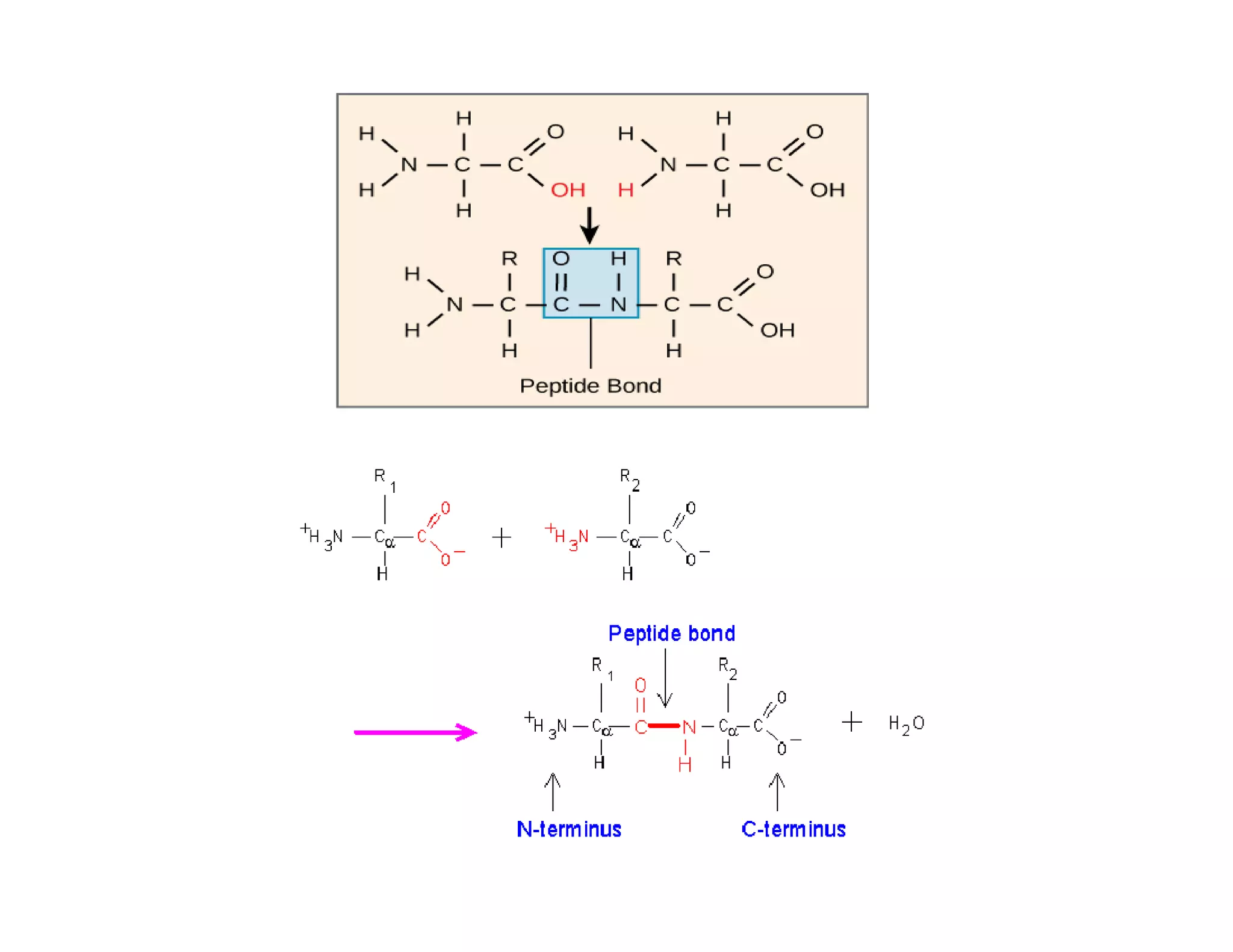
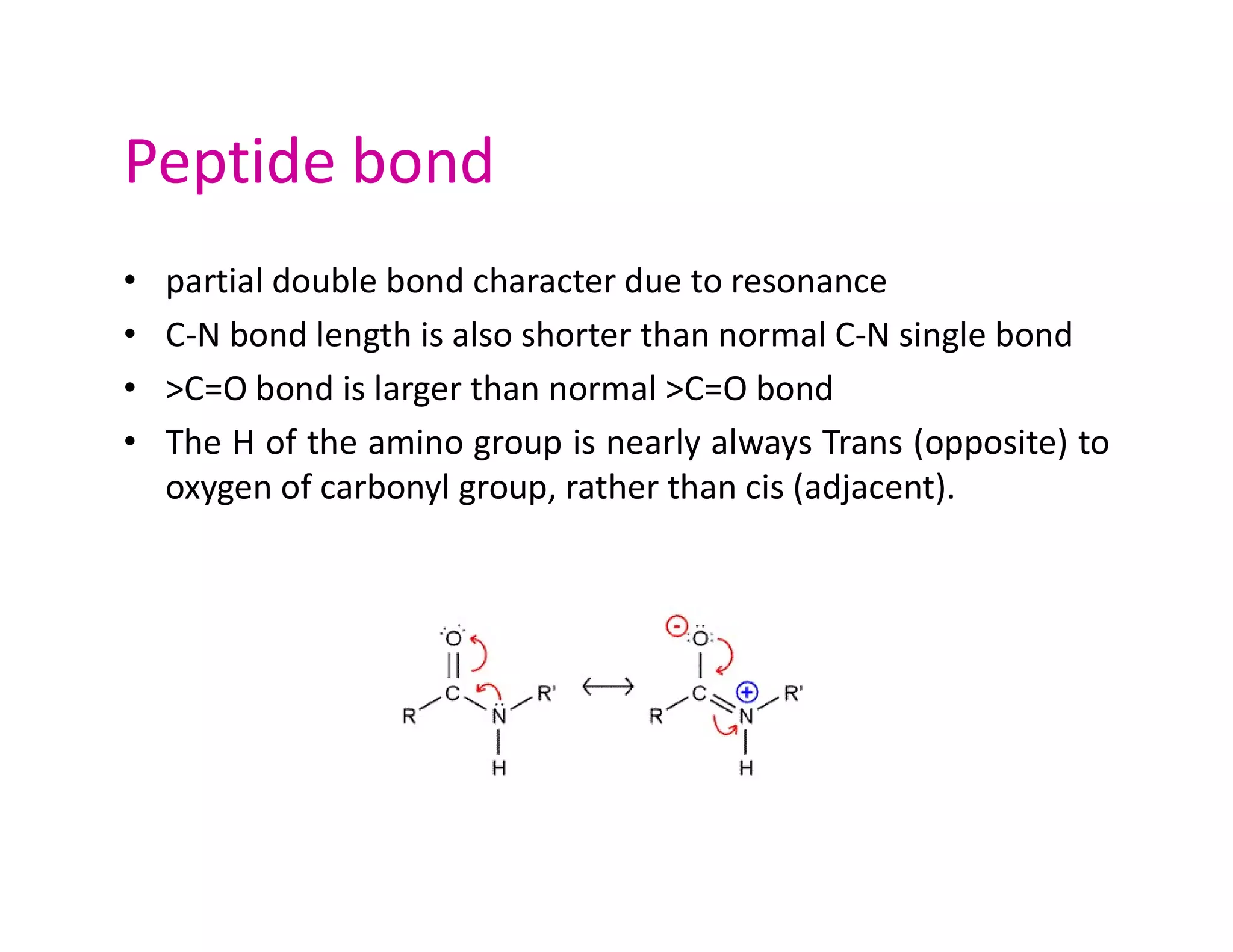
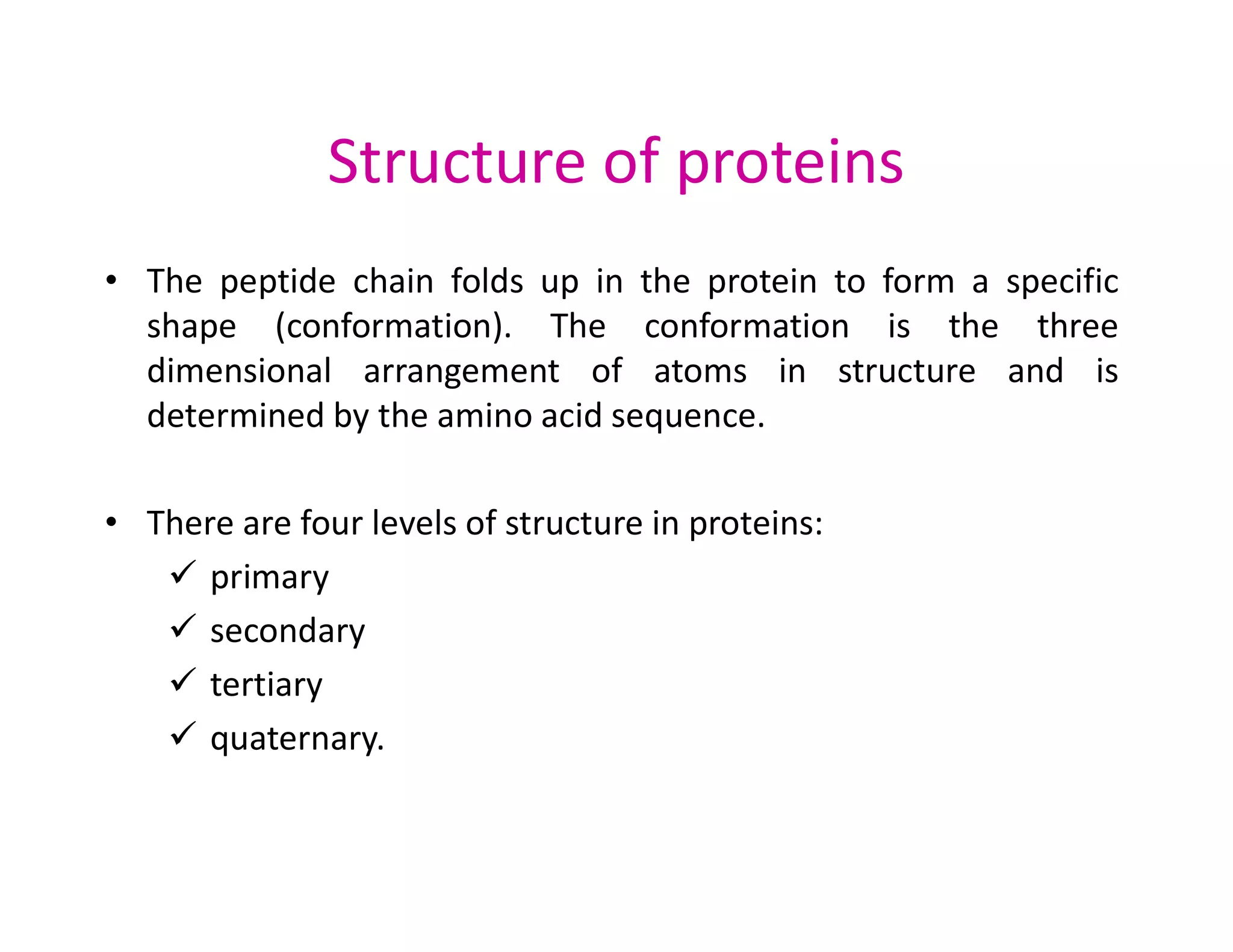
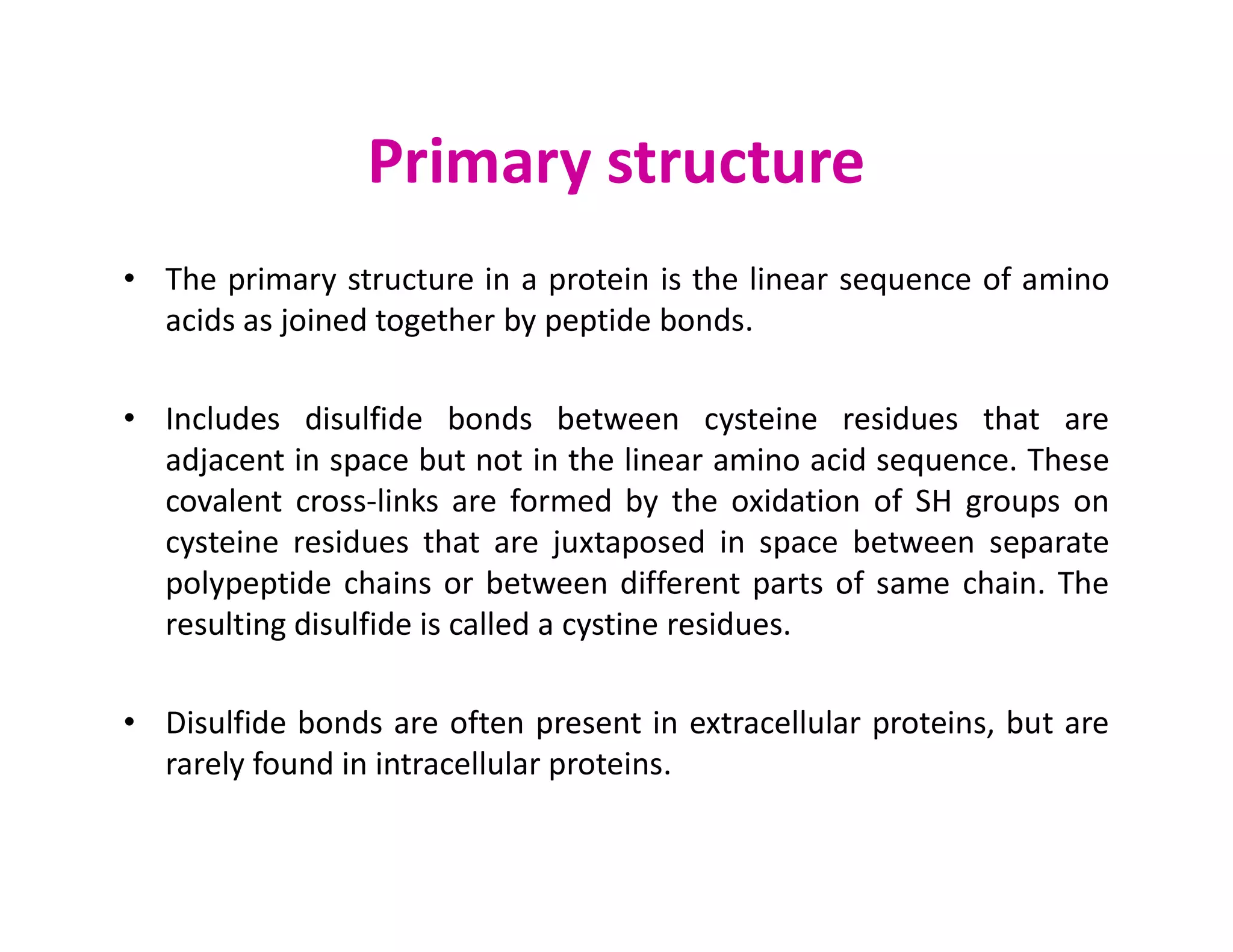
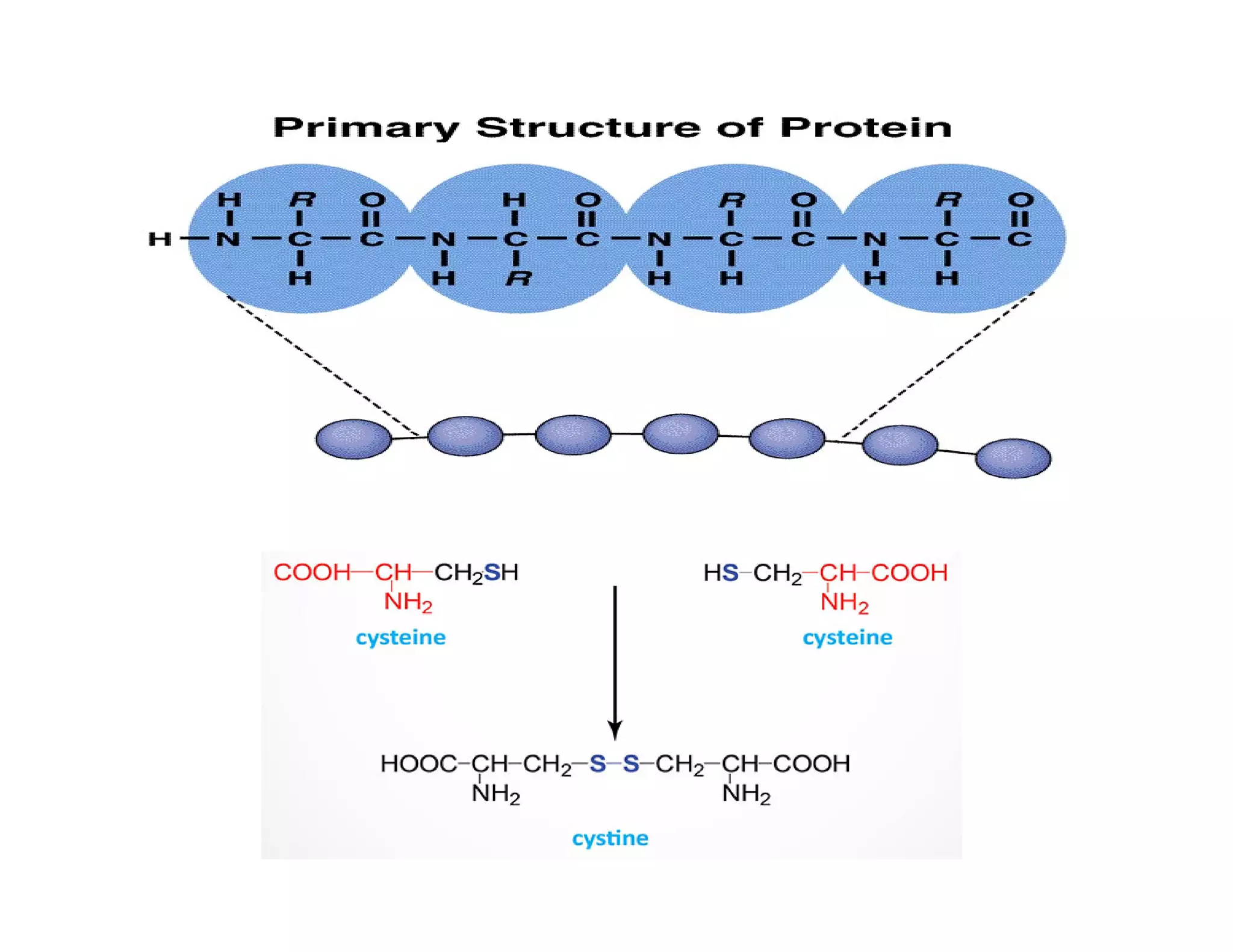
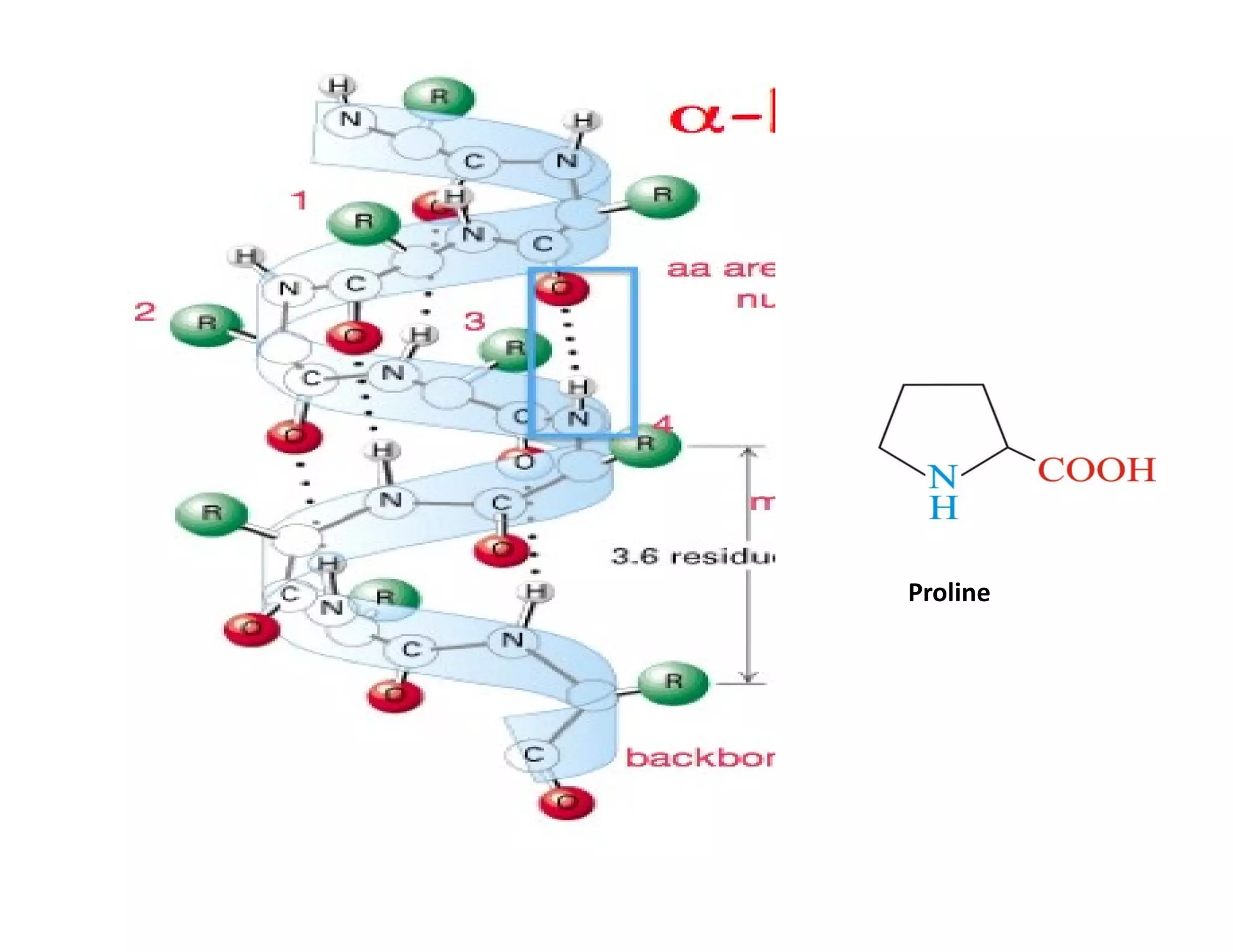
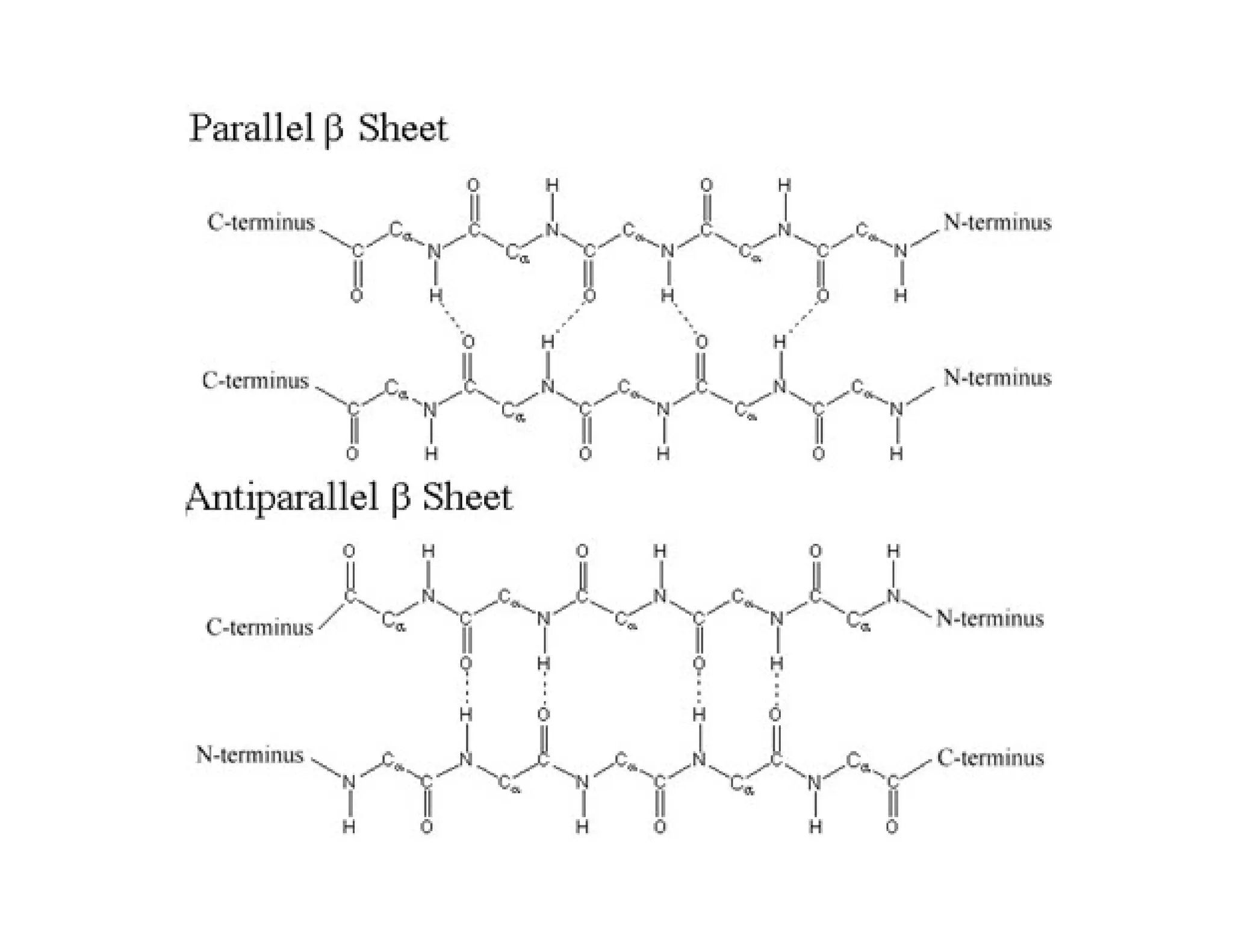
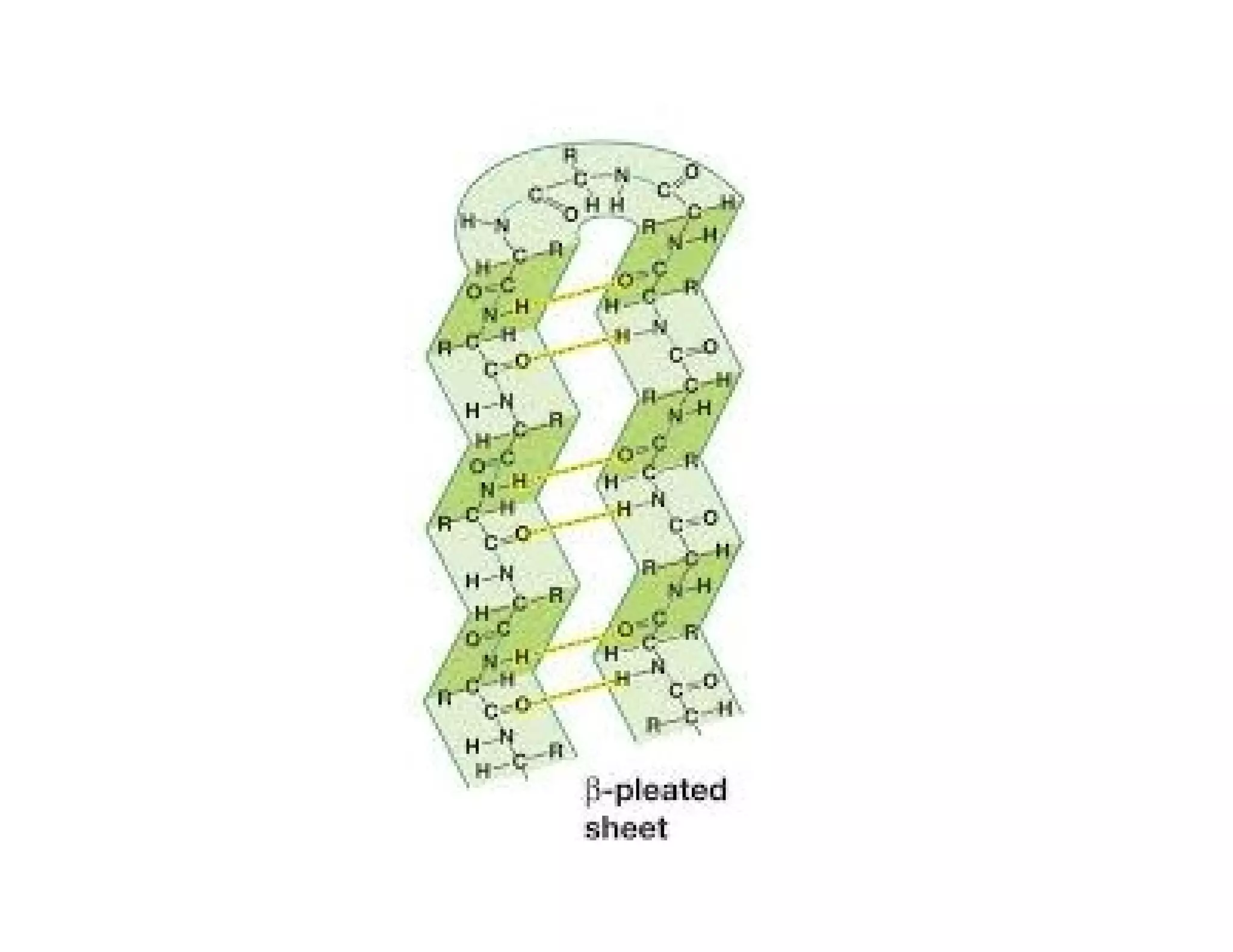
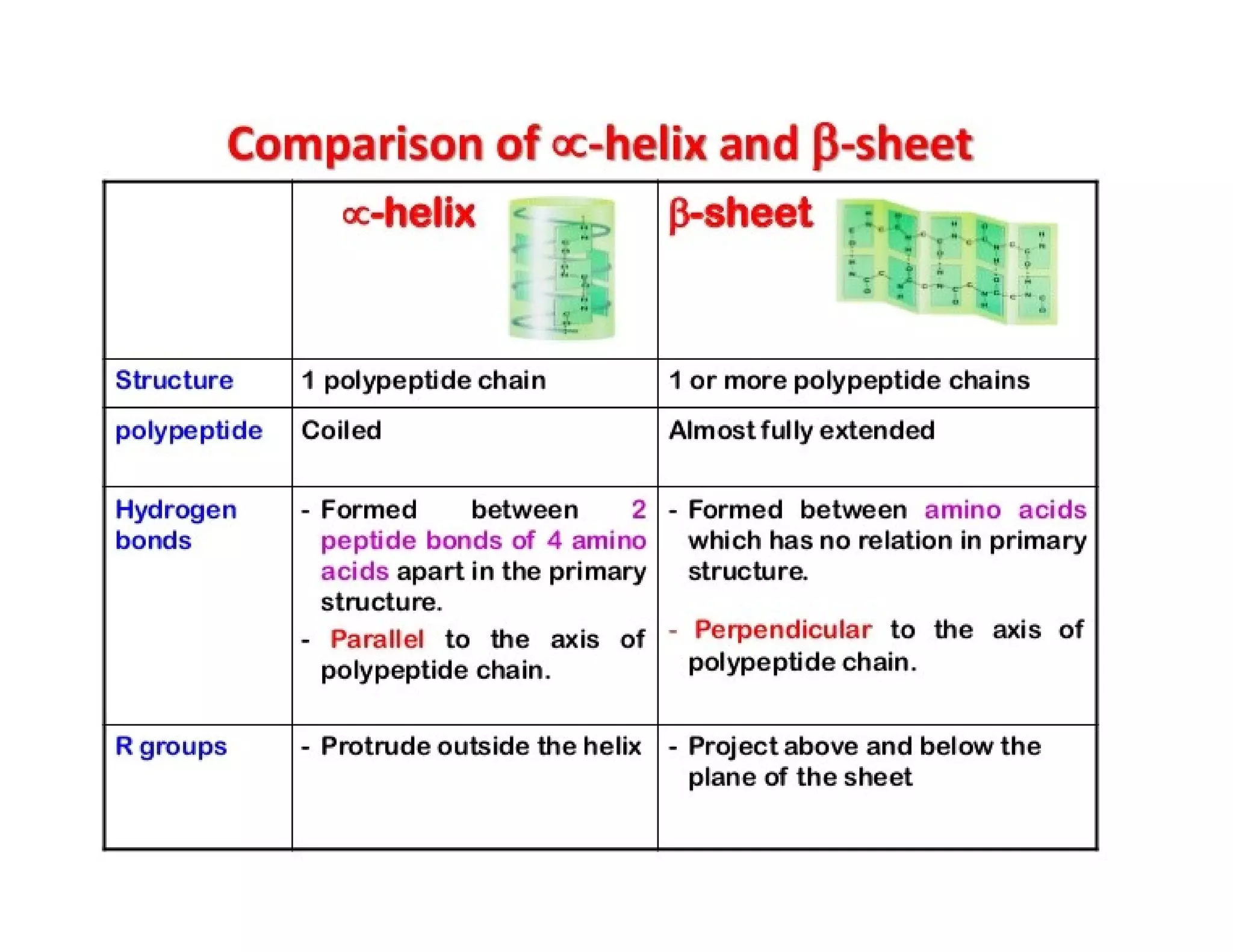
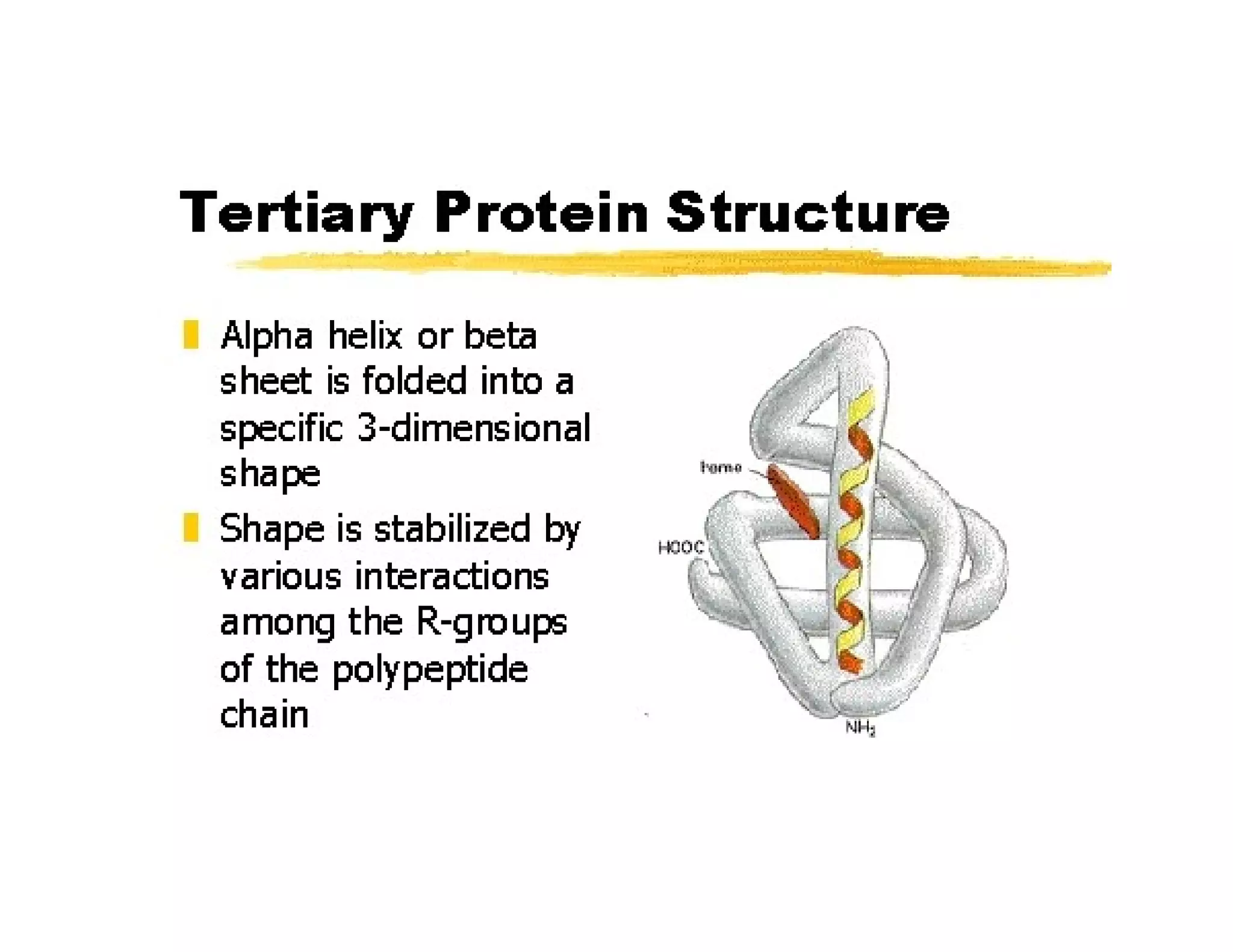
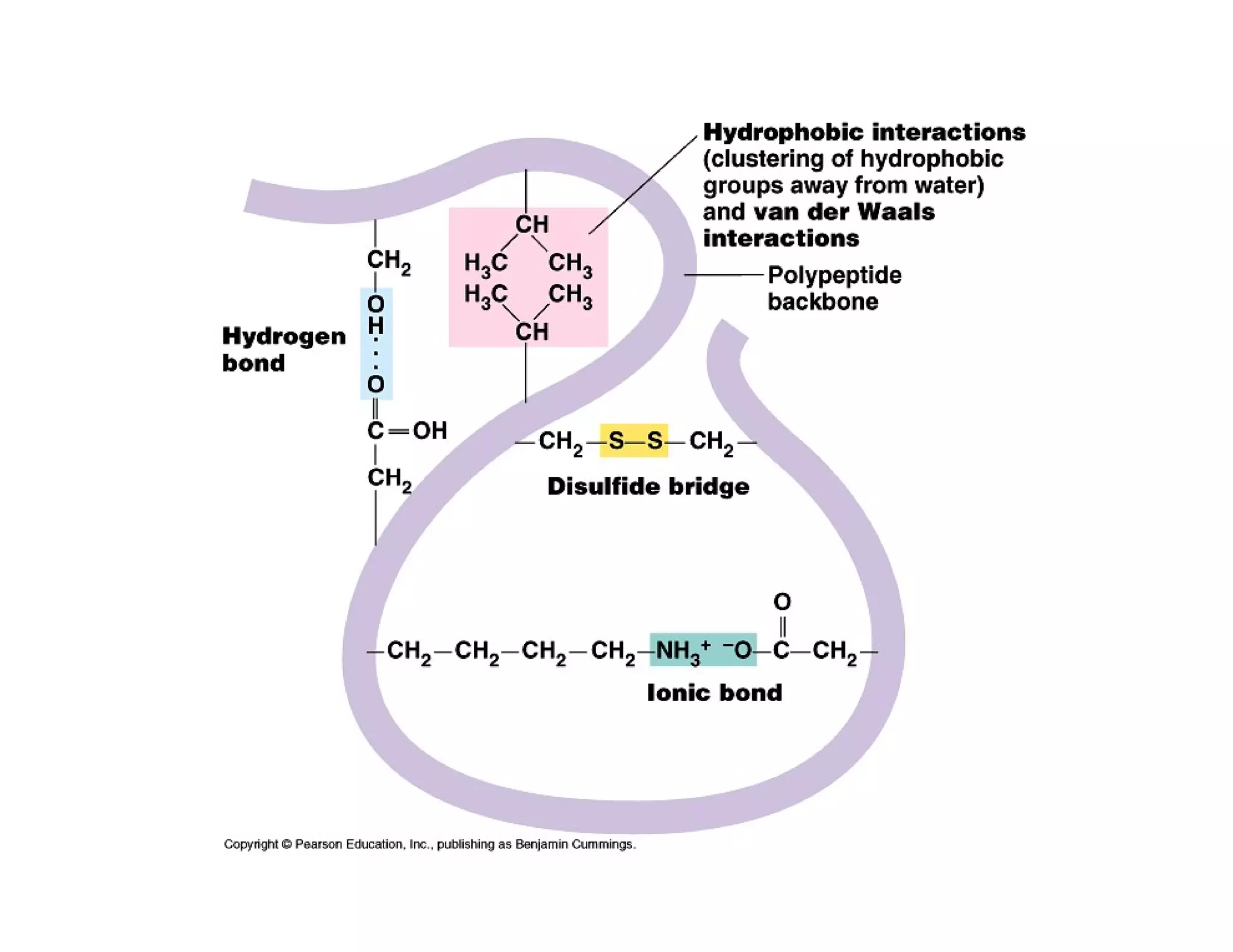
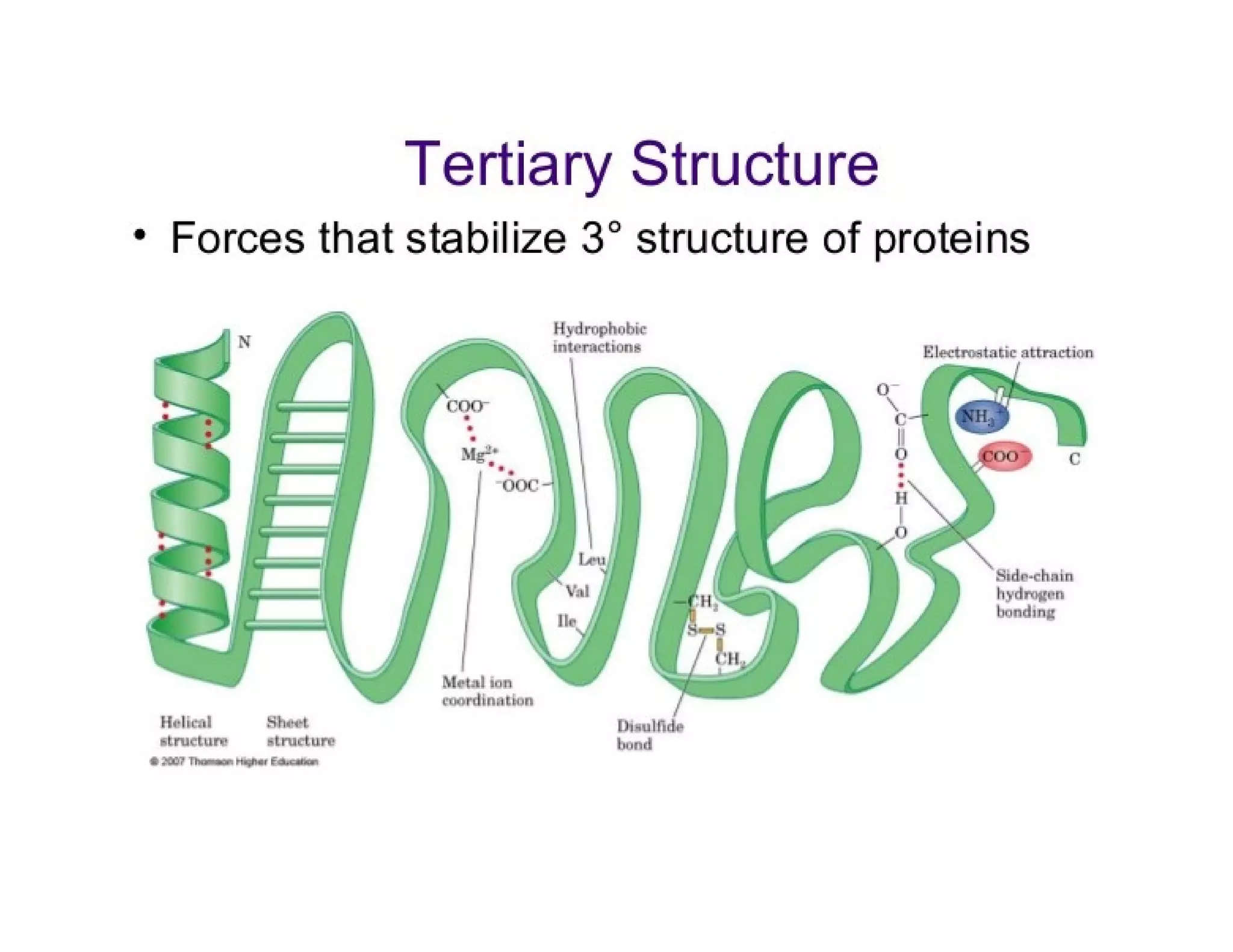
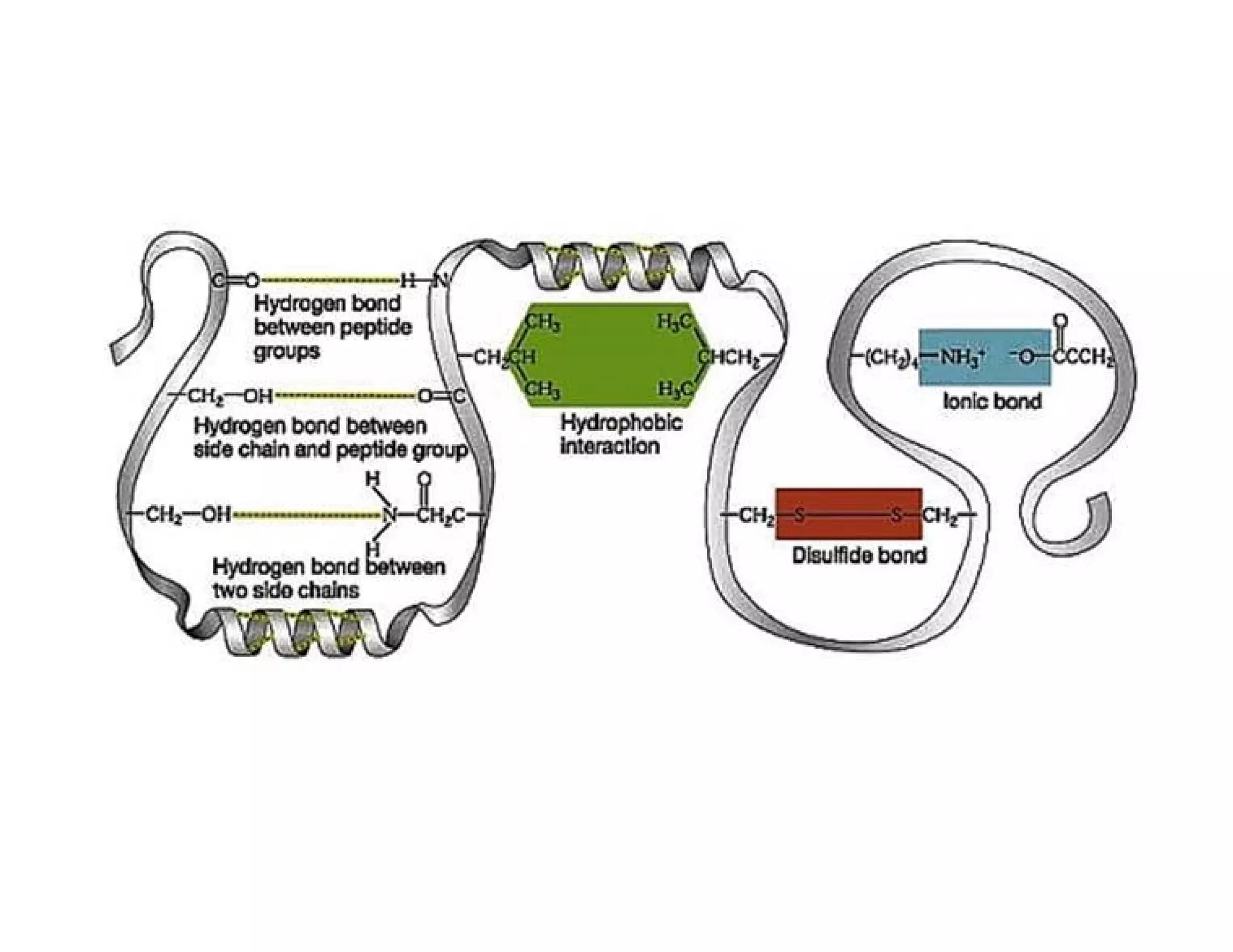
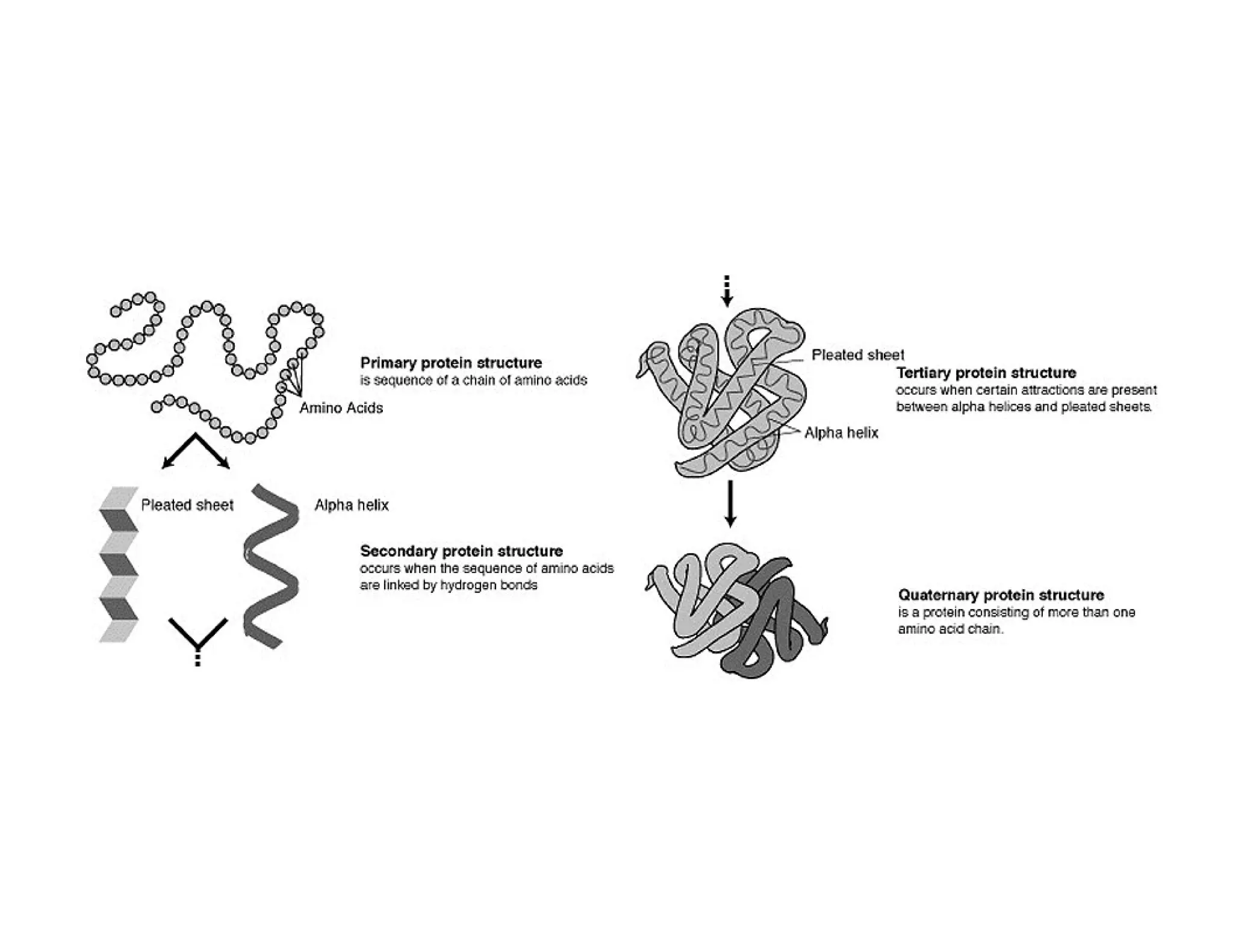
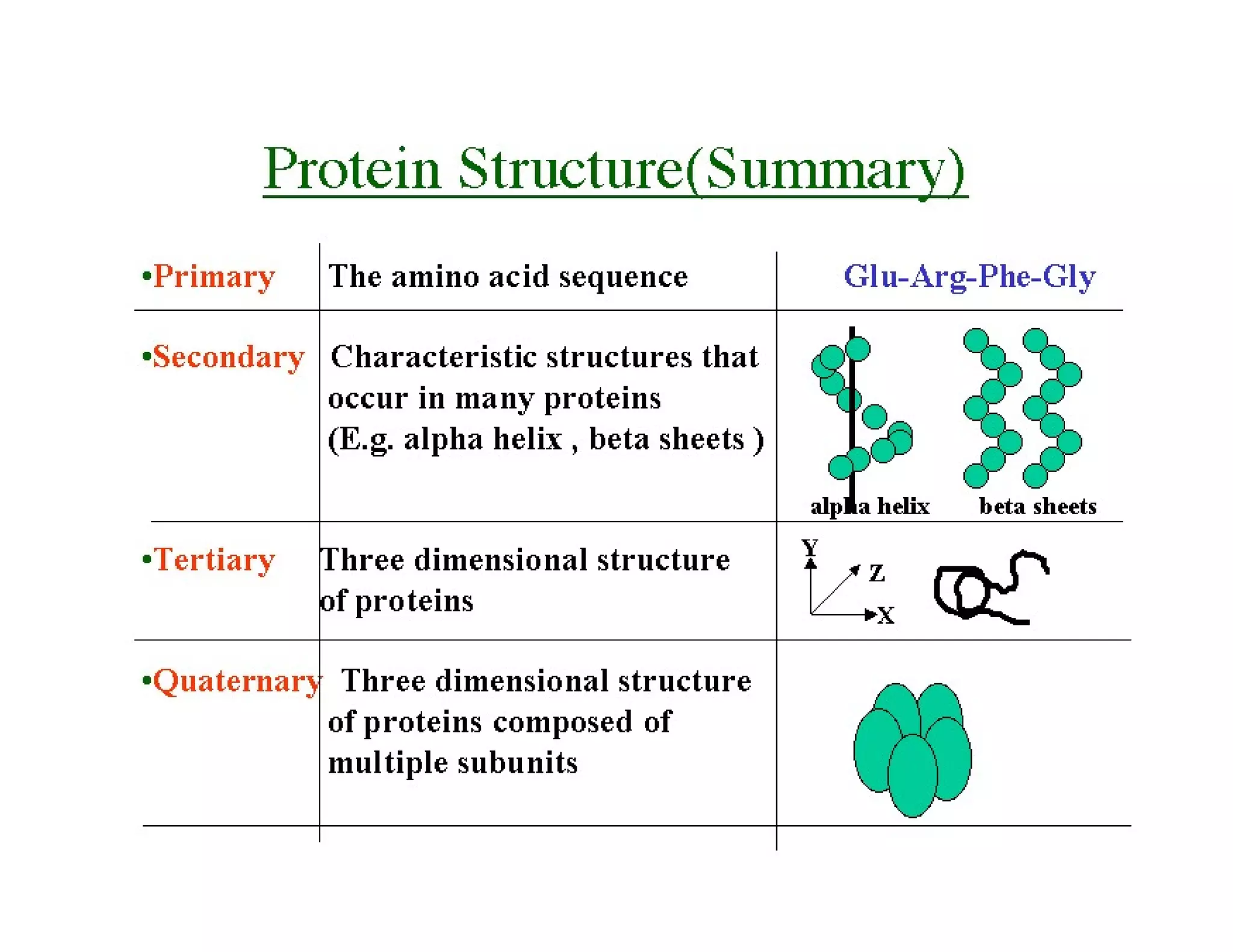
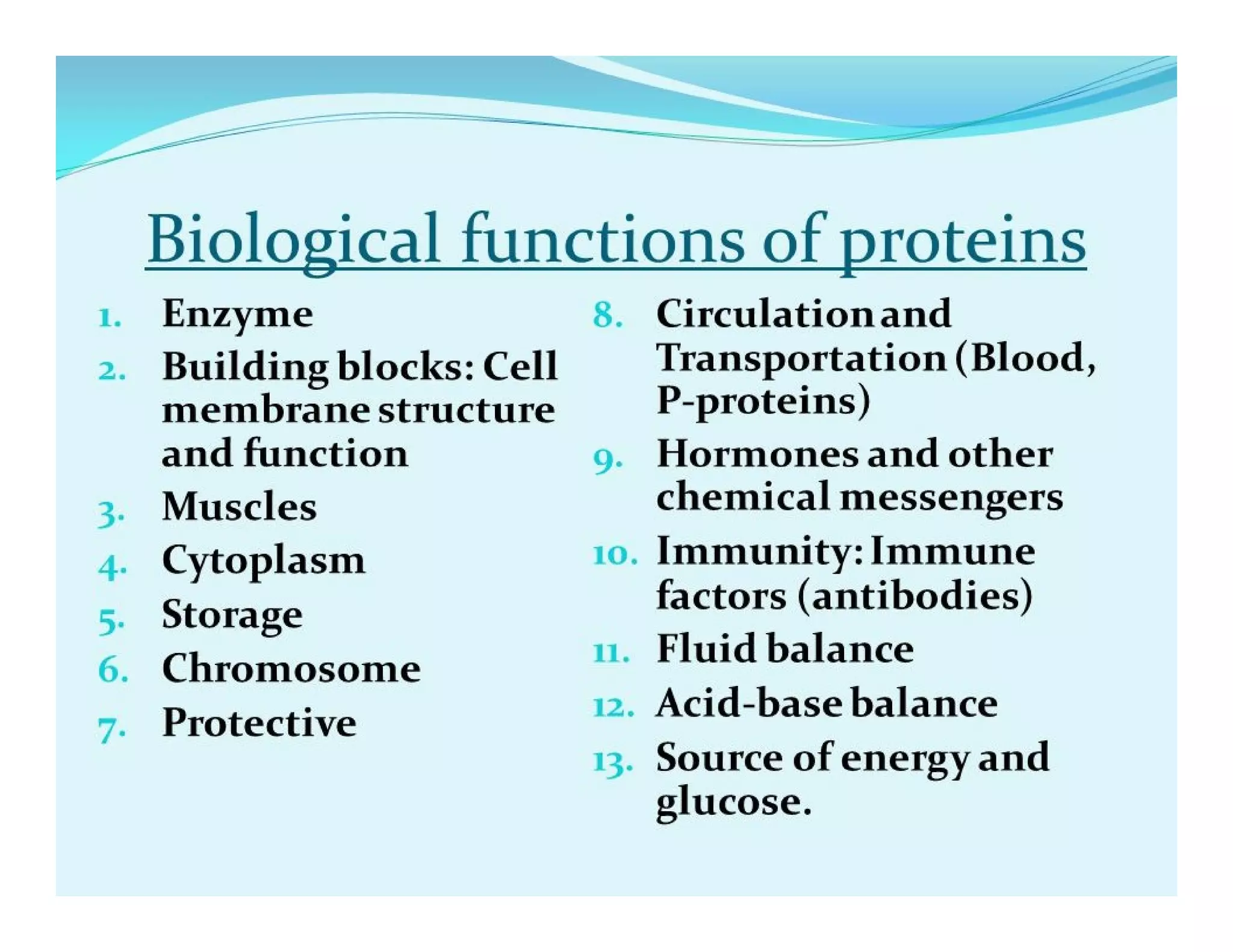
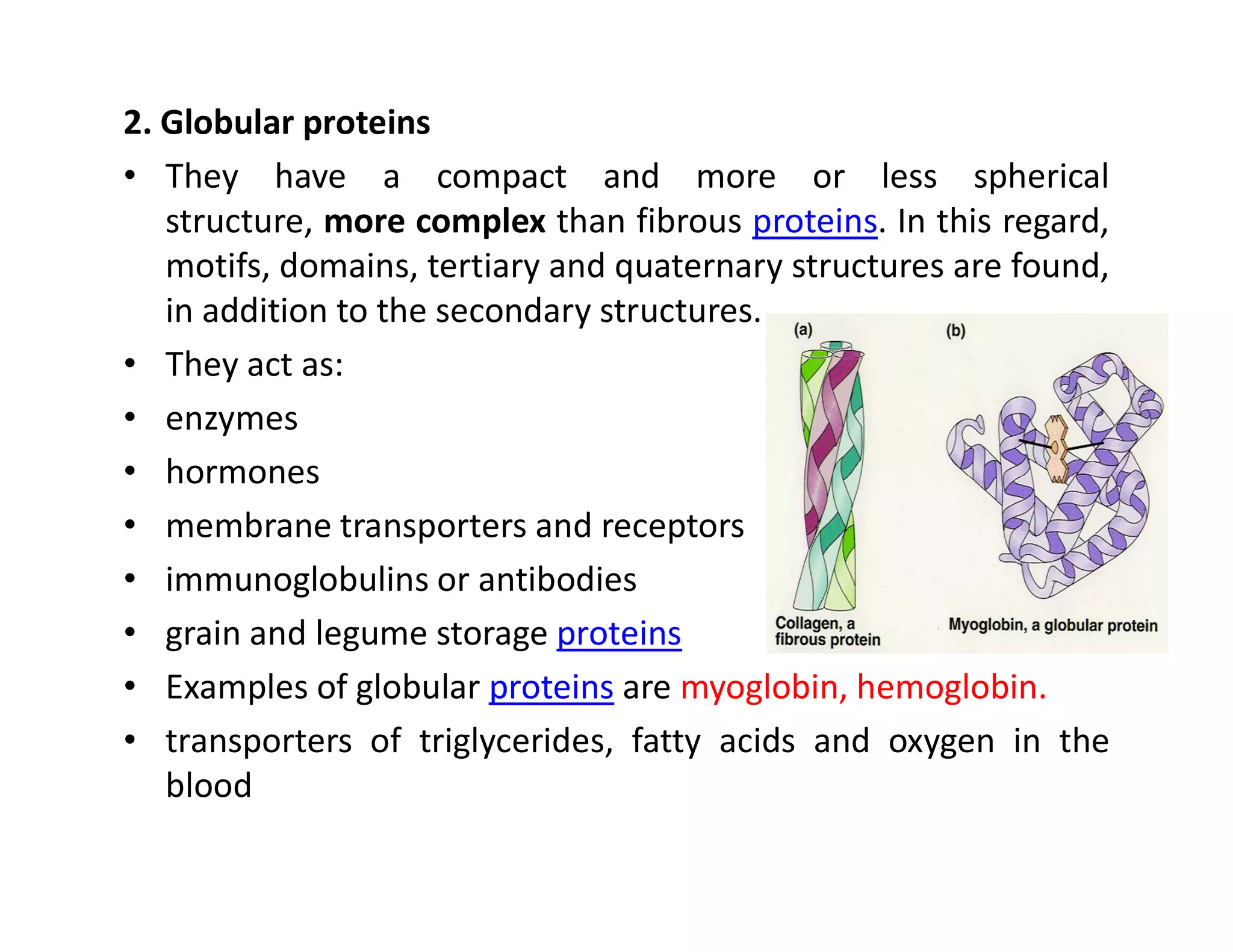
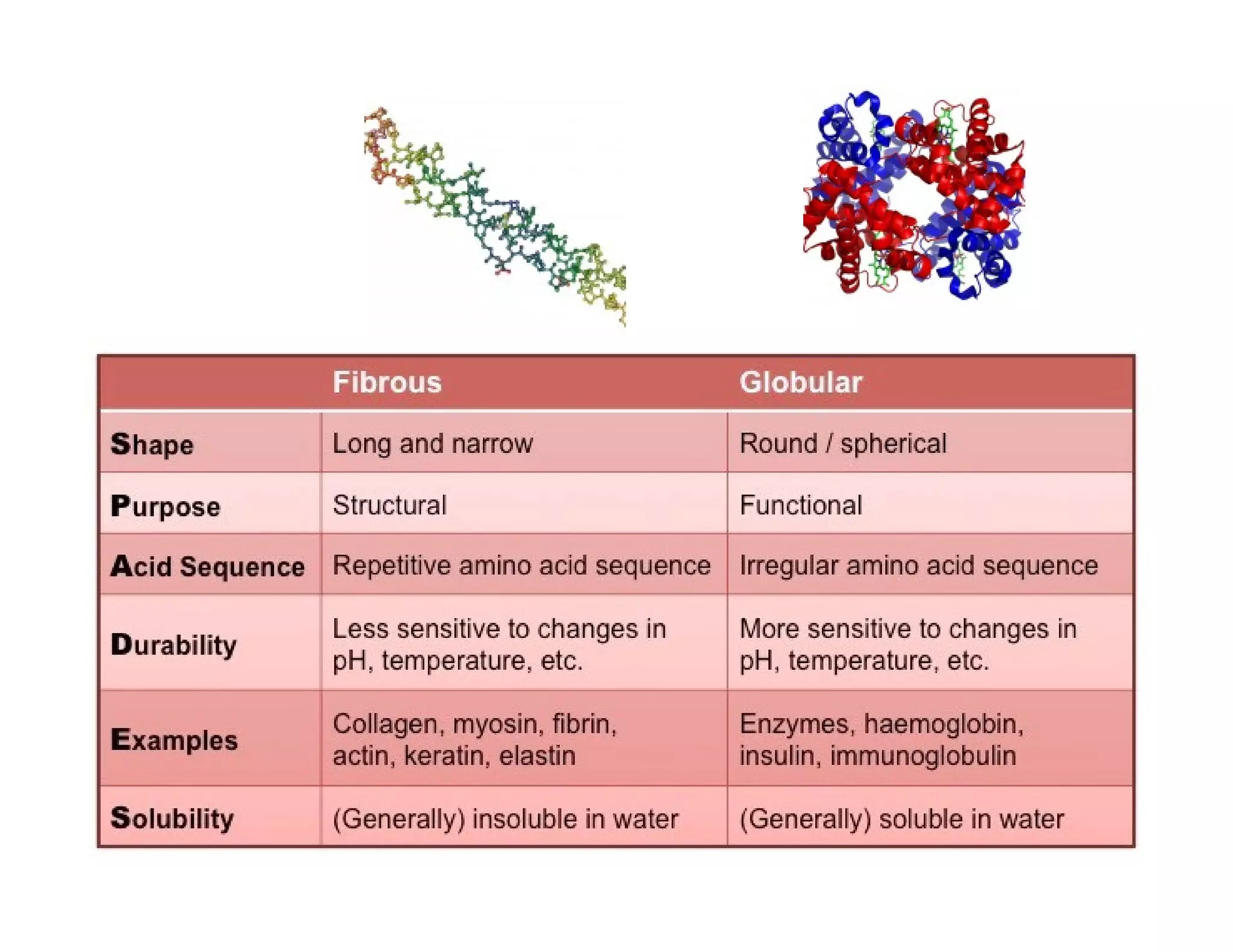
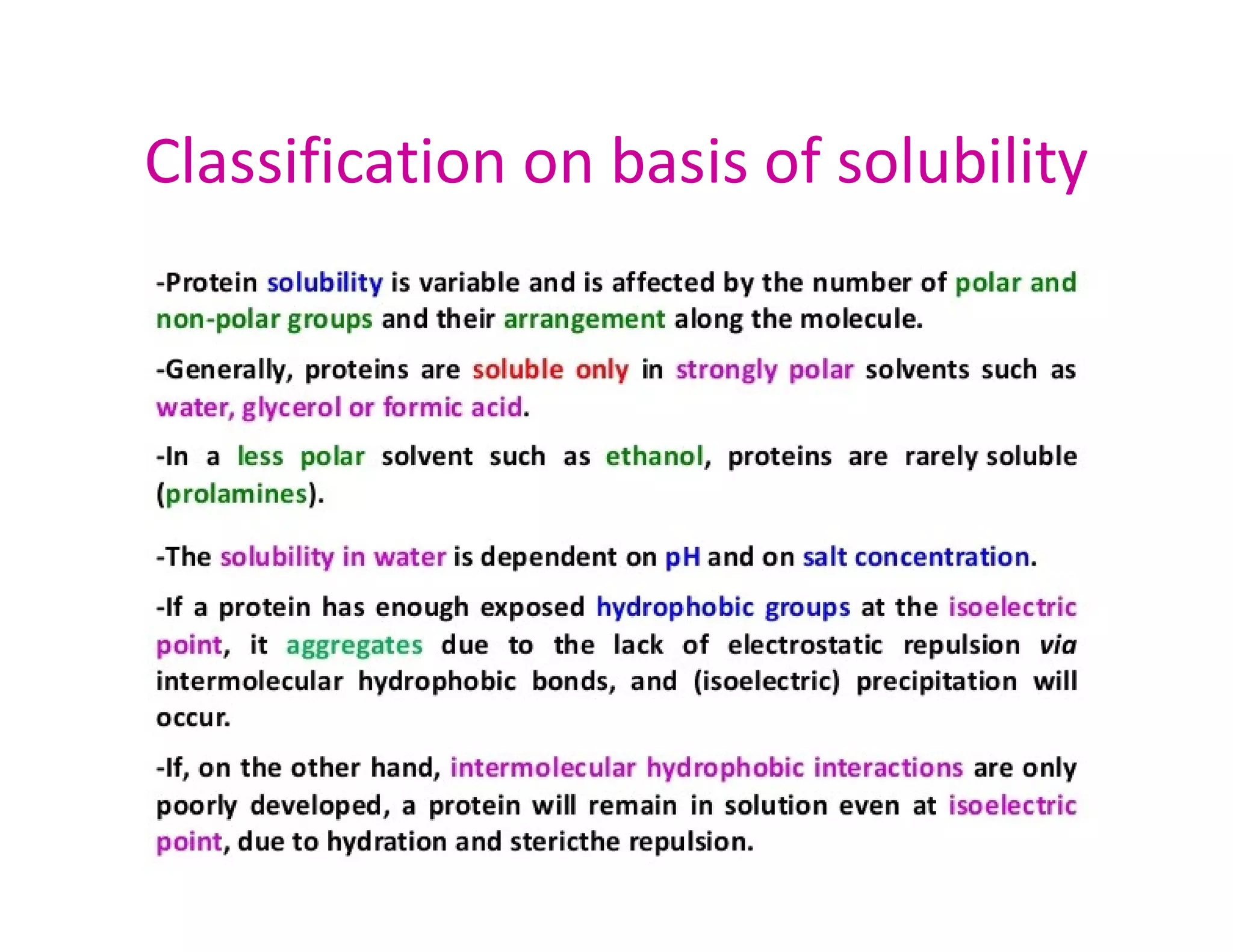
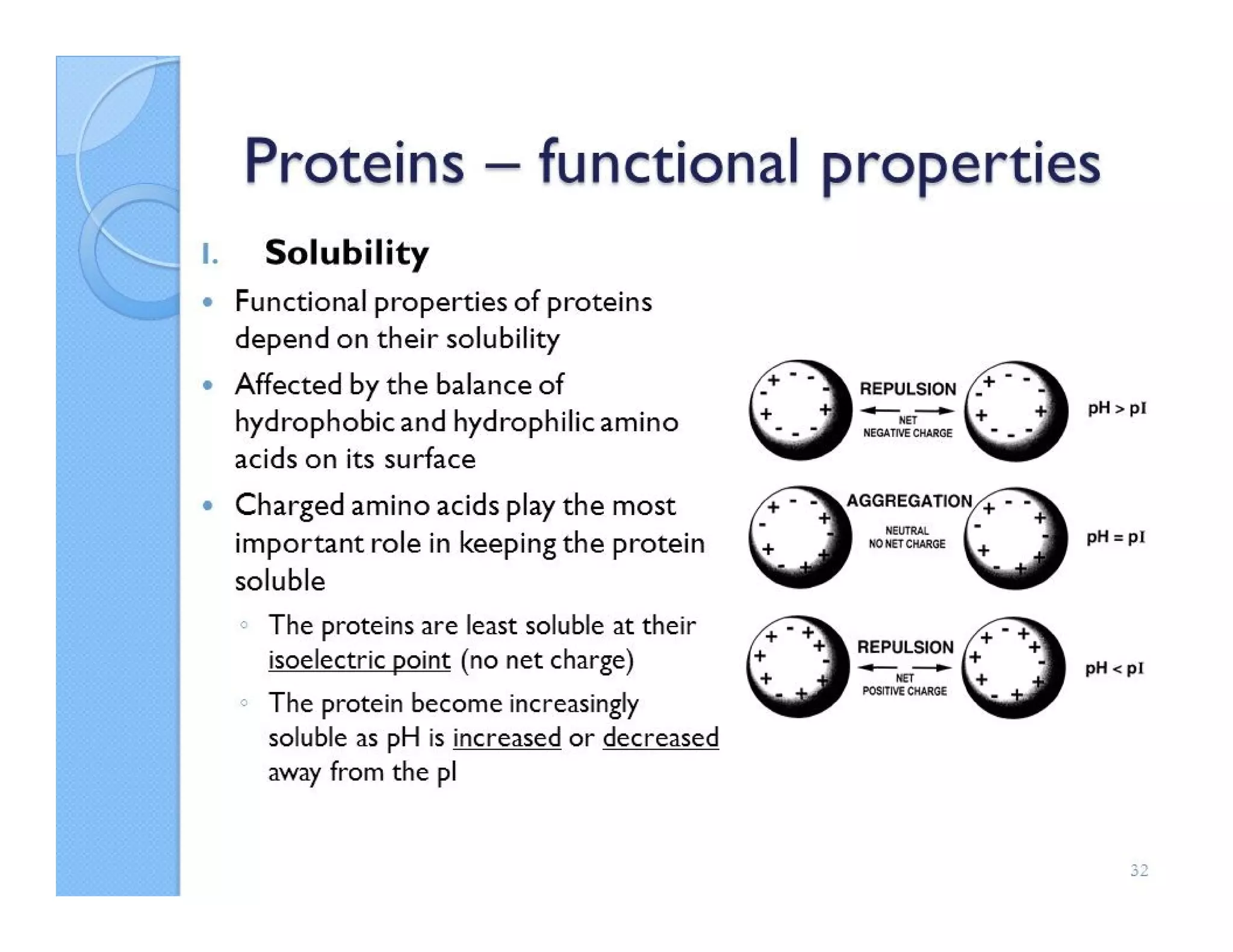
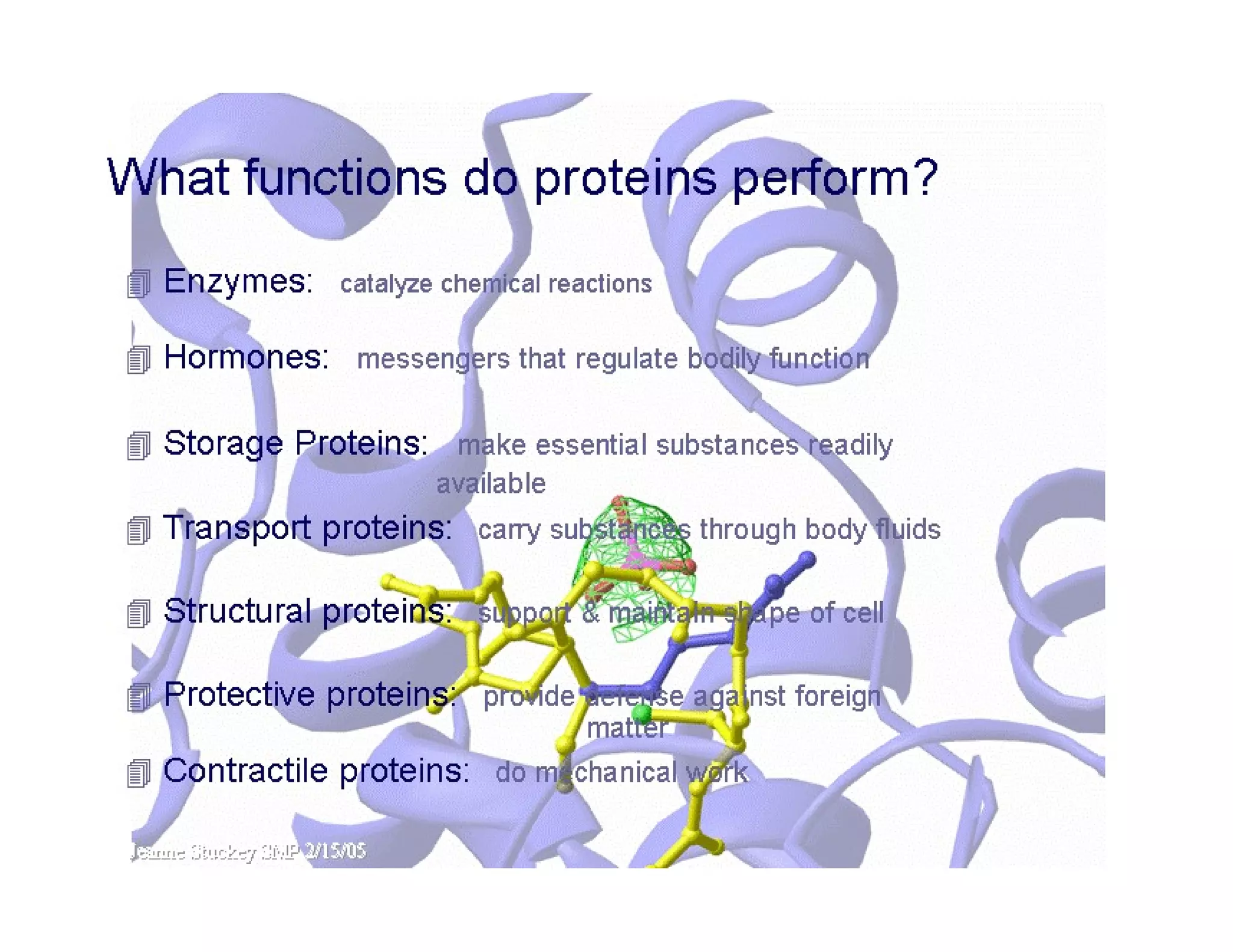
The document discusses the structure, classification, and functions of biomolecules, including proteins, carbohydrates, lipids, and nucleic acids, as well as primary and secondary metabolites. It elaborates on amino acids as the building blocks of proteins, their classification, and the four levels of protein structure: primary, secondary, tertiary, and quaternary. Additionally, it highlights the various functions of proteins, including enzymatic, transport, structural, and hormonal roles.