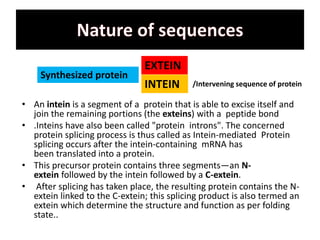
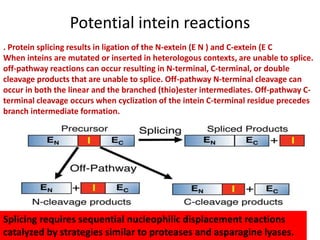
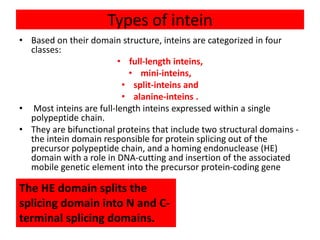
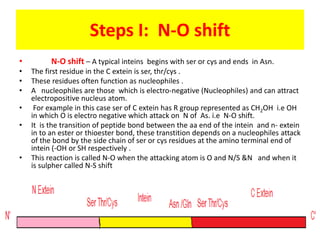
Protein splicing is a post-translational process where non-coding sequences called inteins are removed from precursor proteins, resulting in functional products linked by peptide bonds. Inteins have various classes, such as full-length and split-inteins, and their splicing mechanism involves autocatalytic reactions and specific amino acid sequences that facilitate bond transformations. This detailed process includes key steps like nucleophilic attacks and the formation of thioester bonds, ultimately yielding ligated exteins free of inteins.