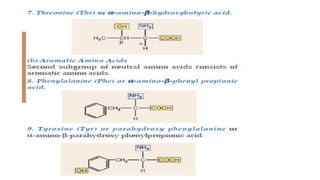
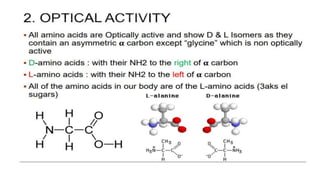
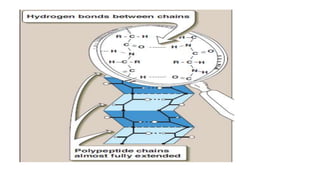
The document discusses proteins and amino acids. It describes that proteins are composed of amino acids, which are the monomers that make up proteins. There are 20 standard amino acids that combine to form various protein structures. The document then classifies proteins based on their shape, size, solubility, physical properties and functions. It provides examples of different types of proteins like keratins, collagens, globulins, and histones. The composition and importance of proteins is also summarized.