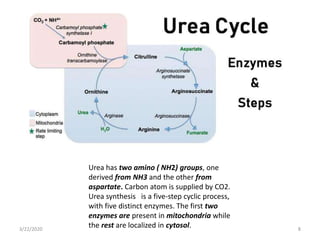
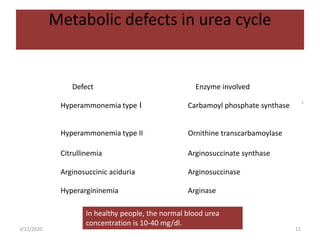
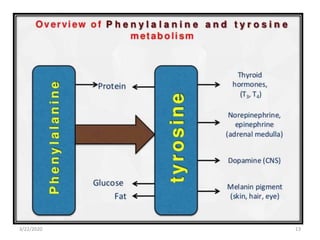
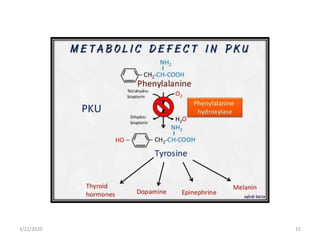
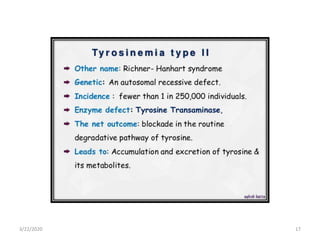
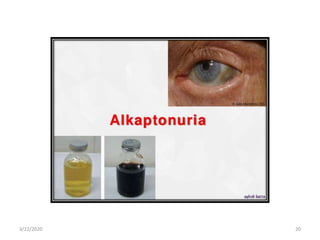
The document discusses amino acid metabolism, covering processes such as transamination, deamination, and decarboxylation, as well as the urea cycle and associated disorders. It details the synthesis and significance of various biological substances and outlines the steps and enzymes involved in the urea cycle, alongside metabolic defects related to it. Additionally, it explores the catabolism of phenylalanine and tyrosine and their related metabolic disorders.