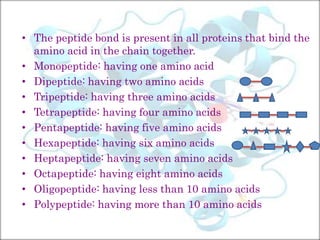
Proteins are macromolecules composed of amino acids linked by peptide bonds. They perform many important functions in the body and are obtained from both animal and plant sources. The document discusses the structure of proteins including their composition of amino acids, classification based on shape and complexity, properties, and sources. It provides a detailed overview of the key aspects of protein structure and function.