The document classifies proteins based on their solubility, shape, composition, and function. It discusses several classifications:
1. Based on solubility and composition, proteins are divided into simple, conjugated, and derived groups. Simple proteins include albumins, globulins, prolamins, glutelins, and others.
2. Based on function, proteins serve as enzymes, hormones, antibodies, storage, transport, toxic, structural, contractile, and secretory functions.
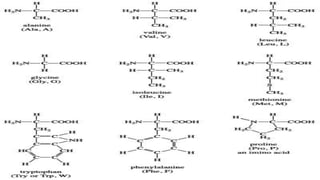
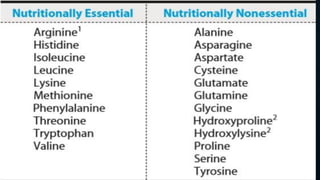
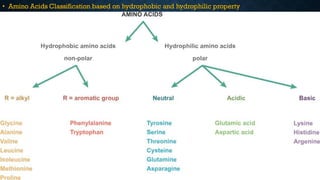
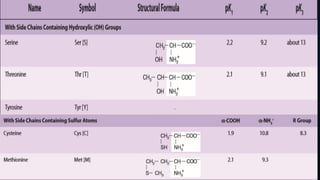
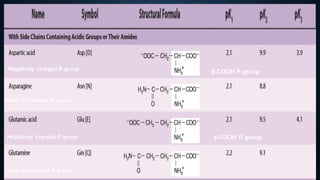
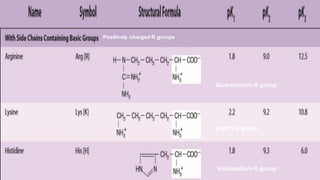
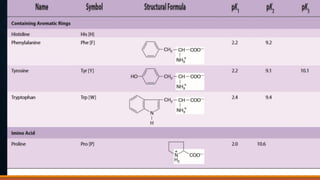
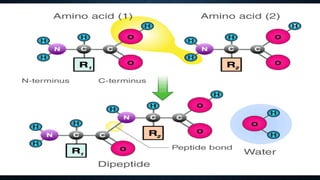
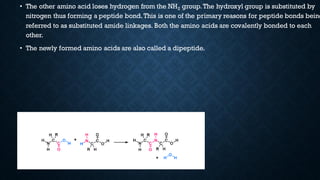
3. Amino acids are the building blocks of proteins. They are classified based on properties like polarity, nutritional requirements, and side chain groups. Peptide bonds form between amino acids and are characterized by their rigidity