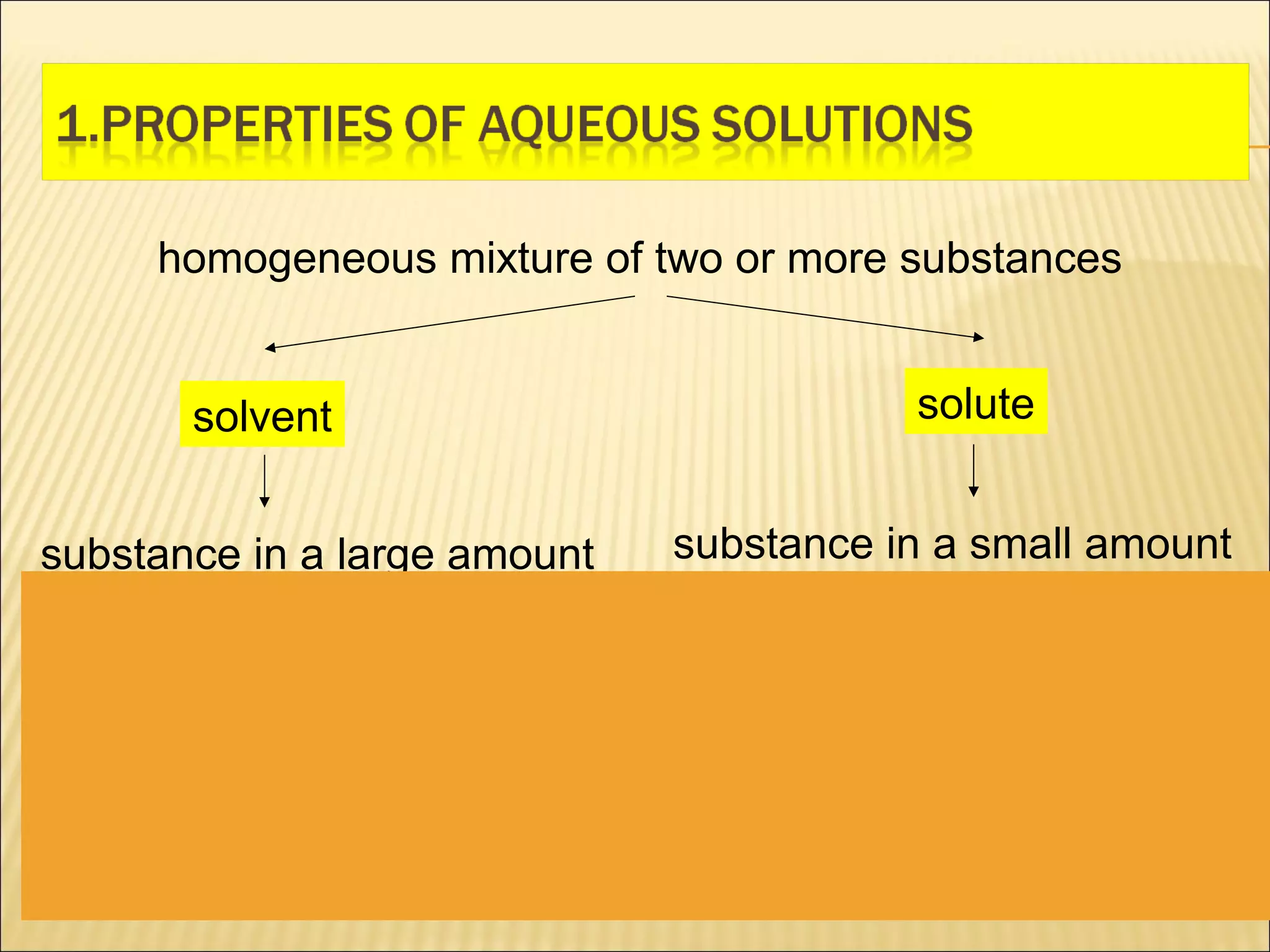
1. The document discusses the properties of solutions including solutes, solvents, electrolytes, and non-electrolytes. Solutions are homogeneous mixtures of substances where one substance is in smaller amounts (solute) and the other is the continuous liquid medium (solvent).
2. The document also covers acid-base reactions and redox reactions that occur in aqueous solutions including precipitation, acid-base, and redox reactions. Precipitation reactions form insoluble products. Acid-base reactions involve proton transfer and redox reactions involve electron transfer.
3. Oxidation states and oxidation numbers are discussed as well as rules for determining oxidation numbers of elements in compounds. Redox reactions involve oxidation, where electrons






![SOLUTION
percentage concentration
% = g [solute] / g solvent X 100
12 g of sodium chloride are solved in 150 g of water.
Calculate the percentage concentration
8%](https://image.slidesharecdn.com/chapter4tro-140131084255-phpapp01/75/Properties-of-Solution-8-2048.jpg)


























![PROPERTIES OF ACIDS
1. acids have a sour taste
vinegar – acetic acid
lemons – citric acid
2. acids react with some metals to form hydrogen
2 HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
EXP8
3. acids react with carbonates to water and carbon dioxide
2 HCl(aq) + CaCO3(s) → CaCl2(aq) + [H2CO3]
H2CO3 → H2O(l) + CO2(g)
4. some acids are hygroscopic
H2SO4 (conc)
EXP9](https://image.slidesharecdn.com/chapter4tro-140131084255-phpapp01/75/Properties-of-Solution-35-2048.jpg)



























































